Abstract
The 8 kDa antigenic protein of Clonorchis sinensis was partially purified by ammonium sulfate precipitation and subsequently by a column chromatographic steps. The purified protein was separated into 7 and 8 kDa protein bands through SDS-tricine gel electrophoresis, while the protein was found to migrate to a 8 kDa band in 7.5-15% SDS-PAGE. The molecular weight of the antigen was estimated to be 110 kDa by Superose 6 HR 10/30 gel filtration. The purified antigen strongly reacted with the human sera of clonorchiasis. The hyperimmune sera of BALB/c mice immunized against the 8 kDa protein were reacted with both the crude extract and the excretory-secretory product of adult worms, but not with the metacercarial extract. Immunohistochemical staining demonstrated that the protein was distributed to the tegument and subtegumental cells and also to the seminal receptacle. The present findings suggest that the 8 kDa protein is a partition of the multicomplex protein originating from various organs of adult C. sinensis, and that it is composed of several 7 and 8 kDa proteins.
-
Key words: Clonorchis sinensis, 8 kDa antigen
INTRODUCTION
Several studies have focused on the detection of specific antigens to improve the diagnosis of human clonorchiasis (
Kim, 1994 &
1998;
Yong et al., 1998;
Hong et al., 1999;
Kang et al., 2001). A number of
Clonorchis sinensis antigenic bands from 8 kDa to 66 kDa reacted to the patient's sera have been identified by immunoblotting. Hong et al. (
1997) reported that 26-28, 34 and 43 kDa bands were the specific and primary targets for clonorchiasis. Hong et al. (
1999) also found that the IgG4 antibody was useful in serodiagnosis for human clonorchiasis and revealed a positive correlation between IgG, IgG4 reaction and infection intensity. Moreover, the IgG4 reaction to 8 kDa band showed no cross-reactions with other trematode infections and antibodies reactive with 8 kDa and 26-28 kDa bands disappeared within 6 months after cure by praziquantel treatment.
Recently, another possible diagnostic protein, 28 kDa glutathione S-transferase was characterized as a useful serodiagnostic antigen (
Kang et al., 2001). Among these diagnostic antigens, the 7 kDa antigen of excretory-secretory products has been recognized to be specific for active infection and to show no cross-reaction with other helminthic infection. Thus, this antigen may act as an indicator for active human clonorchiasis (
Kim, 1998). It had been previously designated as the K2 antigen (
Kim, 1994) and has been the subject of research as a candidate for diagnostic antigens.
As a part of on-going investigations into the diagnostic antigens of C. sinensis, we purified and partially characterized the 8 kDa antigenic protein from the adult worm.
MATERIALS AND METHODS
Preparation of the crude extract of C. sinensis
The metacercariae of
C. sinensis were collected through a peptic digestion of freshwater fish,
Pseudorasbora parva (
Hong et al., 1997). The metacercariae were orally fed to rabbits and adult flukes were collected from their bile ducts after 3 months. For collection of excretory-secretory protein (ESP), some of the fresh adult flukes were incubated in physiological buffered saline at 37℃ for 10 hr and some flukes were homogenized in a teflon pestle homogenizer with Tris-HCl buffer (0.1 M, pH 7.5) containing 0.2 mM iodoacetamide to prevent proteolytic degradation of crude extracts and centrifuged at 15,000 rpm for 1 hr. The resulting supernatant was used as crude extract of
C. sinensis and frozen at -70℃ until used.
The crude extracts were saturated with ammonium sulfate up to a concentration of 80% to induce precipitation of proteins. The precipitated protein was discarded by centrifugation (15,000 rpm, 20 min) and the supernatant was collected, and then dialyzed and concentrated by lyophilization (Hepto-Holten, Denmark). The concentrated protein was dissolved in a small amount of buffer A (20 mM Tris-HCl, 0.15M NaCl, pH 7.4) and dialyzed against buffer A for 10 hr. For purification of 8 kDa protein, gel filtration column was performed using ÄKTA FPLC system (Amersham Pharmacia Biothech, Piscataway, NJ, USA). The dialysate was loaded into a Sephacryl S-100 HR 16/60 column (Amersham Pharmacia Biothech, Piscataway, NJ, USA) previously equilibrated with buffer A. The column was washed with buffer A and the fractions of 8 kDa protein were detected by SDS-PAGE. To determine molecular weight of 8 kDa protein, partially purified 8 kDa protein was loaded into Superose 6 HR 10/30 column with the same buffer. The standard marker proteins were β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), bovine serum albumin (66 kDa) and cytochrome C (12.4 kDa) respectively.
SDS-PAGE and immunoblotting
The purified antigen was analyzed by 7.5-15% gradient SDS-PAGE or separated by 11% SDS-tricine gel electrophoresis (
Schoegger and Jagow, 1987). Resolved proteins in gradient gel were transferred to polyvinylidene difluoride membrane (PVDF, Amersham Pharmacia Biotech, Uppsala, Sweden). The membrane was incubated overnight with 1:100 diluted human sera of clonorchiasis or 1:1,000 diluted a mouse serum immunized with the purified protein. Peroxidase-conjugated anti-human IgG and-mouse IgG (Cappel, Cochranville, PA, USA) were used at 1:1,000 dilution for 3 hr. The blots were developed with 4-chloro 1-naphthol and stopped with distilled water.
The polyclonal antibody was produced using BALB/c mice with the purified 8 kDa protein. Ten BALB/c mice, 6-week old, were immunized with 10 µg purified protein emulsified with an equal volume of Freund's complete adjuvant, followed two weeks later by boosting with incomplete adjuvant. The mice received a boosting intravenous injection of 5 µg antigen and were bled 3 days after final boosting.
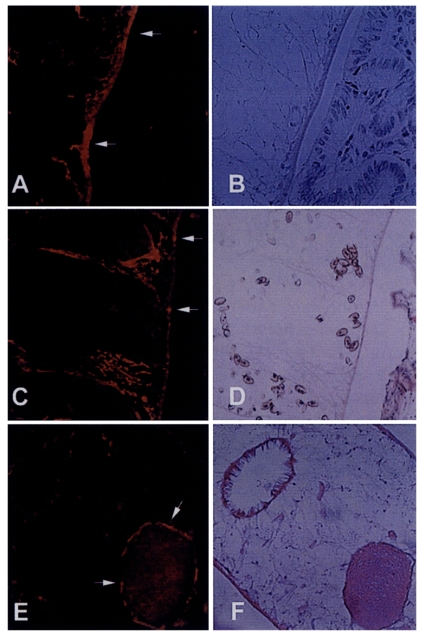
Immunolocalization of the 8 kDa protein
To localize the 8 kDa protein, the formalin fixed liver with the worm was cut into 6 µm thickness. The immune serum against 8 kDa protein was diluted to 1:100 with phosphate buffered saline (10 mM, pH 7.4) for 5 hr and the negative control section was used with pre-immune BALB/c mouse serum (1:100 diluted). The sections were washed twice with the buffer and incubated with tetramethyl rhodamine isothiocyanate-conjugated anti-mouse IgG antibody (Jackson immunoresearch laboratory, PA, USA) diluted 1:50 for 1 hr. The sections were washed twice above buffer and observed with fluorescence microscope Olympus AX 70. The positive reacted sections were then stained with hematoxylin-eosin to visualize and compare the organs reacted with immune sera of 8 kDa protein.
RESULTS
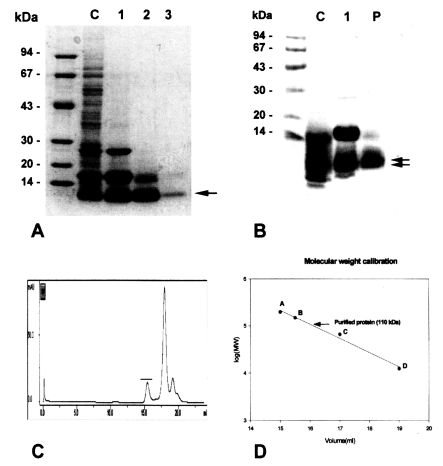
Purification of the 8 kDa protein
The purified 8 kDa protein is presented in
Fig. 1A. The protein was estimated at 110 kDa by Superose 6 HR 10/30 gel filtration (
Fig. 1C & D), but was found to have migrated as a single band to 8 kDa on regular gradient SDS-PAGE (
Fig. 1A). However, the protein was migrated to 7 and 8 kDa with SDS-tricine electrophoresis (
Fig. IB).
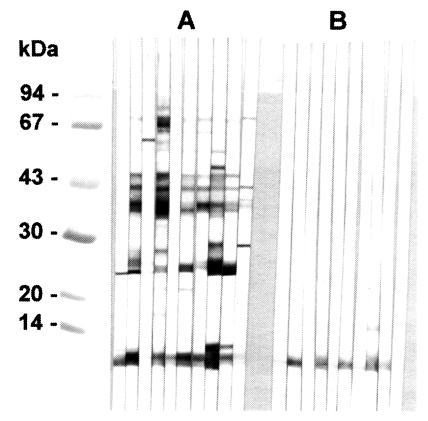
In immunoblotting, the crude extract of
C. sinensis exhibited major antigenic bands of molecular weight 8, 26-28, 34-38, 43 and 70 kDa (
Fig. 2A). The purified 8 kDa protein and the equivalent band of the crude extract both strongly reacted with human clonorchiasis sera (
Fig. 2B).
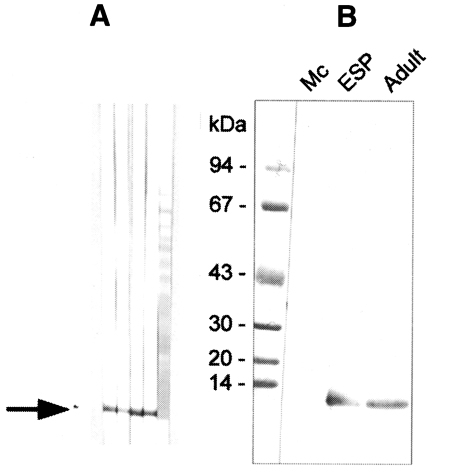
The polyclonal serum from BALB/c mice immunized against the 8 kDa protein was ascertained by immunoblotting to react specifically to the 8 kDa protein band of the
C. sinensis crude extract and of the excretory-secretory product (
Fig. 3A). However, immunoblot analysis demonstrated no reaction to the metacercarial extract (
Fig. 3B). The mouse antibody crossly reacted with approximately 8 kDa band of
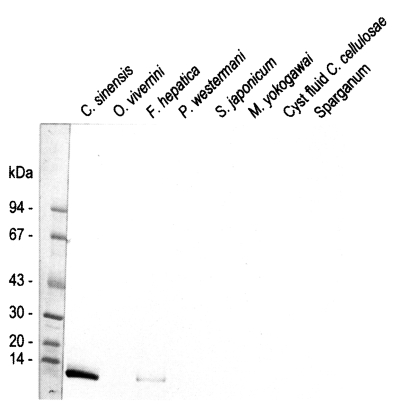
Fasciola hepatica crude extracts while no reaction was observed with the crude extracts of other trematodes or cestodes (
Fig. 4).
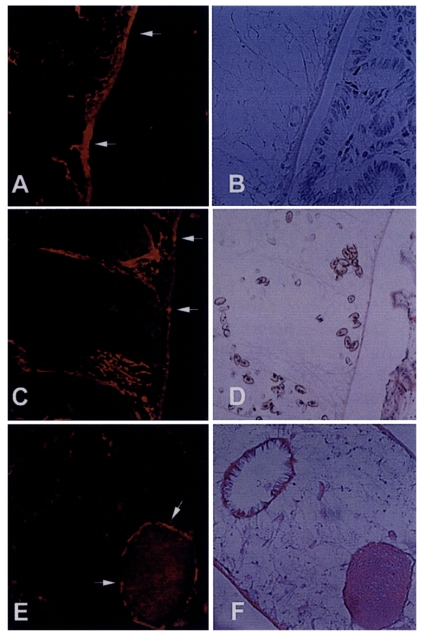
Immunohistochemical staining using the immune serum showed that the 8 kDa protein was distributed at seminal receptacle as well as tegument and the subtegumental cells of the worm (
Fig. 5). The positive reactions were also somewhat observed at parenchymal tissue and some eggs. Pre-immune serum of BALB/c mouse against 8 kDa protein did not react any organs of the worm.
DISCUSSION
The present study confirmed that the 8 kDa protein of C. sinensis is the major antigenic protein in human clonorchiasis. The molecular weight of the 8 kDa protein was estimated to be about 110 kDa on Superose 6 HR 10/30 gel filtration.
Gel electrophoretic studies revealed repeatedly that the protein was presented as an enriched single band on regular SDS-PAGE, but the protein was found to be further migrated to 7 and 8 kDa protein bands on SDS-tricine system (
Fig. 1A & IB). This finding was also observed on a high percentage of SDS-PAGE gradients. It is important to determine whether the 8 kDa protein is one of the degradation by-products of a macromolecular protein. It is quite common for small molecular sized peptides to arise from high molecular structural proteins due to proteolytic activity or through an electrophoresis process (
Sugiyama et al., 1988). Therefore, iodoacetamide (final 0.2 mM), a cysteine protease inhibitor, was added during the extraction of the
C. sinensis crude antigen to prevent proteolytic degradation of the antigen proteins (
Hong et al., 1997). The present results suggest that the 8 kDa protein is one of several smaller subunits, including the 7 kDa molecule, which comprise a larger molecule. The other 7 kDa protein band was previously described as the K2 antigenic band and it was demonstrated that the 7 kDa antigen of adult worm extract or ESP was a valuable indicator in the diagnosis for active human clonorchiasis (
Kim, 1994,
1998;
Kim and Eom, 1998). These results might suggest that the 7 kDa protein in the present study is identical to the molecule previously described by Kim (
1998).
The purification of the 8 kDa protein was difficult because the 7 and 8 kDa proteins comigrated during various kinds of column chromatography and the 17 kDa protein was also co-purified. The purified protein in the present study is predominantly 8 kDa antigen, but it may be mixed with small amounts of the 7 and 17 kDa bands. The protein was present in the enriched soluble fraction at highly saturated ammonium sulfate solution and it had an affinity for hydrophobic ligands such as Phenyl- and Octyl-sepharose CL-4B. This finding suggests that the protein partially possesses a hydrophobic moieties.
From immunoblotting analysis it was evident that the 8 kDa protein of the crude extract and its purified form reacted well with the human sera of clonorchiasis (
Fig. 2). The serum reaction to the antigen band has previously been described as very stable and consistent (
Kim, 1998;
Hong et al., 1999). Several other proteins such as the 26, 28, 34-38, 43 and 70 kDa bands of the crude extract were also observed. Hong et al. (
1999) had demonstrated that the 8 kDa protein is the major antigenic band in the reaction with IgG and IgG4 subclass. They also found that the 8 kDa band of crude extracts showed no cross-reaction with the sera of other trematode infections and disappeared within 6 months after the completion of treatment. Similar findings have also been made by other studies (
Kim and Eom, 1998;
Kim, 1998).
Immunoblotting assay using the polyclonal antibody with crude antigens of other helminthes identified the 8 kDa protein band of the crude extract of
C. sinensis and
F. hepatica (
Fig. 4). This antibody cross-reacted only with the 8 kDa protein of
F. hepatica, not with that of other trematodes or cestodes. This finding implies that the two trematodes partly share the antigenic epitope of the 8 kDa protein. Moreover, this antibody confirmed that the protein was rich in the crude extract and also the ESP of adult worms, but not in the metacercarial extract (
Fig. 3B). These findings indicate that the 8 kDa protein is produced at the adult stage. It was observed in our results that the protein was distributed at the tegument layer and the tegumental cells and in the seminal receptacle (
Fig. 5). Such a location is in good agreement with the finding of high concentration of the protein in the ESP because the tegument is the main secreting sites of the worm. Especially the tegumental cells were found to include the protein in the cytoplasm. This fact suggests the protein is produced in the tegumental cells and secreted through the tegument.
Since the 8 kDa protein of C. sinensis was found to be located in the outer and inner borderline of the body, it may play a role in various metabolic processes on the body surface.
The present findings suggest that the 8 kDa protein is a partition of the excretory or secretory multicomplex protein of adult C. sinensis. The protein is composed of 7 and 8 kDa protein which share a fairly similar properties, and it may be involved in metabolic processes on the tegument.
Notes
-
This study was supported in part by a grant (#Nl-02-01-A-05) from the Ministry of Science and Technology, 1997-2000.
References
- 1. Hong ST, Kho WG, Lee M, Lee JS, Lee SH. Immunoblot pattern of clonorchiasis. Korean J Parasitol 1997;35:87-93.
- 2. Hong ST, Lee M, Sung NJ, et al. Usefulness of IgG4 subclass antibodies for diagnosis of human clonorchiasis. Korean J Parasitol 1999;37:243-248.
- 3. Kang SY, Ann IY, Park CY, et al. Clonorchis sinensis: molecular cloning and characterization of 28-kDa glutathione S-transferase. Exp Parasitol 2001;97:186-195.
- 4. Kim SI. Immune reactions between excretory-secretory antigens and specific antibodies of Clonorchis sinensis before and after praziquantel treatment in experimentally. Korean J Parasitol 1994;32:35-42.
- 5. Kim SI. A Clonorchis sinensis-specific antigen that detects active human clonorchiasis. Korean J Parasitol 1998;36:37-45.
- 6. Kim SI, Eom KS. Immunoblot analysis of human clonorchiasis after praziquantel treatment. Korean J Immunol 1998;20:245-248.
- 7. Schoegger H, Jagow GV. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 1987;166:368-379.
- 8. Sugiyama H, Horiuchi T, Tomimura T. Antigenic characteristics of larval Paragonimus westermani. Jpn J Parasitol 1988;50:169-174.
- 9. Yong TS, Yang HJ, Park SJ, et al. Immunodiagnosis of clonorchiasis using a recombinant antigen. Korean J Parasitol 1998;36:183-190.
Fig. 1Purification of 8 kDa protein from Clonorchis sinensis crude extracts. A, Purified 8 kDa protein was analyzed on 7.5-15% gradient SDS-PAGE. Lane C, crude extracts; lane 1, supernatant of ammonium sulfate saturation; lane 2, partially purified 8 kDa fraction through Sephacryl S-100 HR column; lane 3, purified 8 kDa protein. B, Analysis of the purified protein by SDS-tricine system. Lane C, crude extracts; lane 1, partially purified 8 kDa; P, purified 8 kDa. The 8 kDa protein was separated to 7 and 8 kDa proteins (arrows). C, Superose 6 HR 10/30 gel filtration of partial purified 8 kDa protein. The 8 kDa protein was eluted at fraction of volume 15.5 ml (indicated by bar). D, Estimation of molecular weight of the 8 kDa protein. The protein was estimated to be 110 kDa through the Superose 6 HR column. Standard marker proteins were capitalized. A, β-amylase (200 kDa); B, alcohol dehydrogenase (150 kDa); C, bovine serum albumin (66 kDa); D, cytochrome C (12.4 kDa).
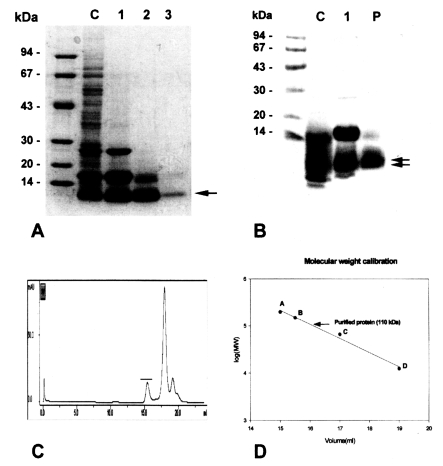
Fig. 2Immunoblotting of crude extracts and 8 kDa protein with clonorchiasis sera. A, Crude extracts of C. sinensis. B, Purified 8 kDa. Electrophoresis was performed on 7.5-15% gradient gel.
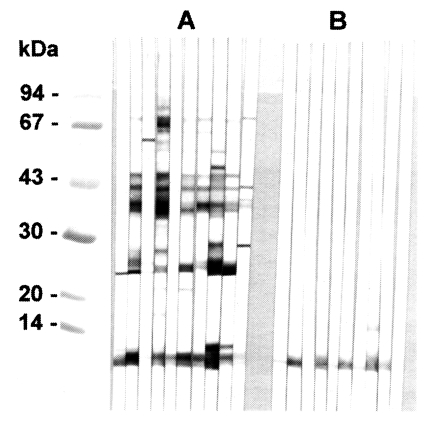
Fig. 3
A, Production of anti-8 kDa polyclonal antibody. BALB/c mice were used as described in materials and methods. B, Immunoblotting of crude extracts of metacercariae, adult and adult ESP with anti-8 kDa polyclonal antibody. The antibody was able to recognize the 8 kDa protein of adult and ESP, but not that of metacercariae.
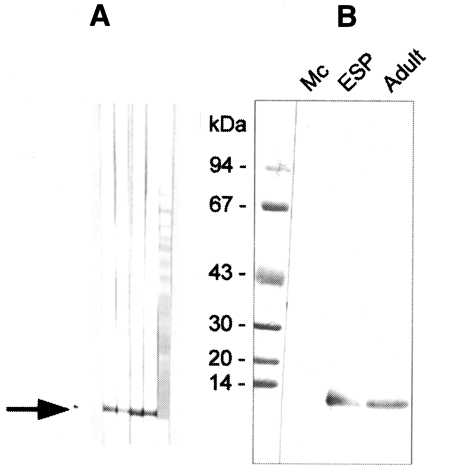
Fig. 4Reactivity of anti-8 kDa polyclonal antibody with proteins of human-infecting other parasites. The antibody demonstrated cross-reaction with crude extracts of Fasciola hepatica.
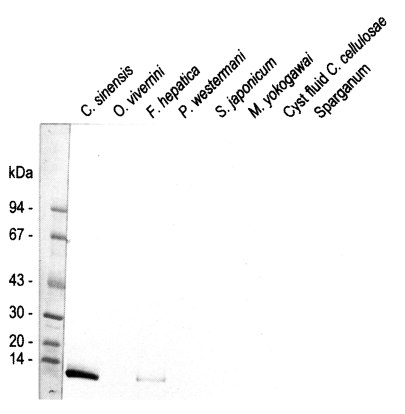
Fig. 5Immunolocalization of 8 kDa protein with immune serum. A and C, The protein was distributed at the tegument and subtegumental cells (arrows). Eggs were also slightly reacted with the anti-8 kDa antibody. Right panels B & D showed the same slide with hematoxylin-eosin staining. E, Seminal receptacle of the worm was also strongly reacted with the antibody. F, Hematoxylin-eosin staining of the slide E.