Abstract
We investigated population densities of mosquitoes infected with sporozoites in three highly epidemic areas of Josan-ri and Jangpa-ri (Paju City) and Dongjung-ri (Yeoncheon County) in Korea. Anopheline mosquitoes were collected from both indoors and outdoors by human baiting collection method during the period of the first week of June to the second week of September 1999. Total 13,296 female mosquitoes were collected and 8,650 (65.1%) were Anophelines. Thirty seven percent (3,199) of the Anopheline mosquitoes were captured outdoors and 63.9% (5,531) indoors. Employing a sandwich enzyme-linked immunosorbent assay (ELISA), we analyzed a total of 7,820 Anopheline mosquitoes and found that 7 Anopheline mosquitoes were infected with sporozoites. The positive rate in Josan-ri was 0.14% (5/3,500) and 0.15% (2/1,370) in Jangpa-ri. The total positive rate in all three surveyed areas was 0.09% (7/7,820). The mosquitoes infected with the sporozoites were detected on June 28th (n=2), July 5th (n=1), July 19th (n=1), August 9th (n=1), September 6th (n=1), and the last one on September 13th (n=1). They were all classified as Anopheles sinensis, which showed positive reaction in ELISA test. Therefore it might be concluded that Anopheles sinensis plays an important role in re-emerging malaria transmission in Korea.
-
Key words: Plasmodium vivax, sporozoite rate, ELISA, Anopheline
INTRODUCTION
Until the mid-1970's, tertian malaria (
Plasmodium vivax) was prevalent throughout Korea (
Soh et al., 1985). However, as a result of the National Malaria Eradication Program with help from the World Health Organization (WHO), the incidence of malaria rapidly decreased (
Ministry of Health and Social Affairs, 1966;
Paik et al., 1988). The two cases in 1984, which were relapsed after a long incubation period, were the last indigenous malaria recorded in Korea (
Soh et al., 1985). In 1993, however, a young soldier who had never been abroad and served within the demilitarized zone (DMZ) was diagnosed to be vivax malaria (
Chai et al., 1994). Subsequently, in 1994 two civilians were also reported as having vivax malaria (
Cho et al., 1994). Indigenous malaria patients had thereafter increased annually by three to five times until 1998. It was believed that the spread of malaria in Korea had reached a plateau. There were total 16,436 malaria cases reported from 1993 to 2001 (unpublished data, The Ministry of Health and Welfare, Korea).
It is well known that seven species of Anopheline mosquitoes exist in Korea, such as
Anopheles sinensis,
An. yatsushiroensis,
An. lesteri,
An. koreicus,
An. pullus,
An. sineroides, and
An. lindesayi. Among them, only two species,
An. sinensis and
An. yatsushiroensis have the ability to transmit malaria parasites in Korea (
Ree et al., 1967;
Hong, 1977).
An. sinensis was found to be the dominant species during malaria transmission seasons and was confirmed as a main vector (
Ree et al., 1967;
Hong, 1977;
Lee et al., 2000). As for
An. yatsushiroensis, one out of 1,469 mosquitoes dissected was infected with oocysts of
P. vivax (
Hong, 1977). Malaria transmission is ultimately dependent upon the sporozoite rate: the proportion of female Anopheline mosquitoes with sporozoites in their salivary glands. Many factors determine sporozoite rates in natural mosquito populations, including mosquito susceptibility, population densities, feed habits, longevity, duration of extrinsic malaria cycle, and other host-vector parameters (
MacDonald, 1952). In this study, we investigated densities of sporozoite infected mosquitoes in the areas that are well known as highly epidemic malaria areas.
MATERIALS AND METHODS
Study areas
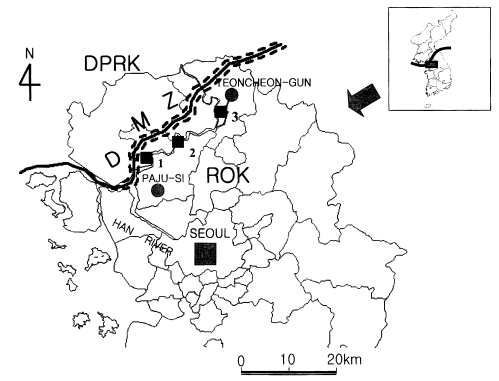
This study was conducted near the DMZ in Korea, and mosquitoes were collected around the villages, Josan-ri (37°56'30"N, 126°41'05") and Jangpa-ri (37°56'30"N, 126°50'30"E) of Paju City and Dongjung-ri (38°4'20"N, 126°57'20"E) of Yeoncheon County. Josan-ri, Dongjung-ri and Jangpa-ri lies to the 2 km, 4 km and 7 km of DMZ, respectively (
Fig. 1).
Mosquitoes in all three areas were collected every week described above from June to September 1999. Collections commenced at 19:00 and continued until 02:00 the following day. In order to find out the proportion of captured mosquitoes according to their blood sucking habits, mosquitoes were collected from both indoors and outdoors. For the collection of the outdoors, one of two collectors exposed his bare arms and legs, while the other aspirated the mosquitoes on the body. For indoor collections, we set a camping tent and kept its back and front door open and proceeded like the outdoor collection. Mosquitoes were sorted and classified into different species based on taxonomic criteria. Only Anopheline mosquitoes were analyzed in this study.
ELISA for detection of P. vivax sporozoites in mosquitoes
After proper classification, mosquitoes were placed in 1.5 ml microfuge tube according to different species and collection places and frozen at -20℃ until use. Mosquitoes were ground with pestles in 50 µl blocking buffer (BB, 10 g BSA; 5.0 g Casein; 0.1 g Thimerosal; 0.01 g Phenol red; 1,000 ml phosphate buffered saline (PBS), pH 7.4) with Nonidet P-40 (NP40, 5 µl of NP40/1 ml of BB). The pestle was also rinsed twice with 75 µl volumes of BB (Total = 200 µl). Mosquitoes were tested individually by ELISA method in 96-well soft microtiter plates (Dynatech Laboratories, USA). Briefly, 50 microliters of monoclonal antibody of P. vivax CSP-210 type (0.025 µg) and P. vivax CSP-247 type (0.1 µg) were added to each well and incubated overnight at room temperature. After aspirating the monoclonal antibody solution and incubating plates filled with 200 µl BB for 1 hr, the plates were aspirated and 50 µl of each homogenized mosquito extract was added to each type of monoclonal antibody coated well plates. After 2 hrs, the wells were washed twice with PBS-Tween 20 (0.05%). Horseradish peroxidase (HRP)-conjugate monoclonal antibody (0.05 µg/50 µl of BB for P. vivax CSP-210 type; 0.1 µg/50 µl of BB for P. vivax CSP-247 type) was then added to each well plates were washed 3 times with PBS-Tween 20 after 1 hr incubation. Finally, 100 µl of 2.2'-azino-di-(3-ethyl-benzthiozoline-6-sulfonic acid) (ABTS) peroxidase substrate (Kirkegaard & Perry, USA) was added to each well and the plates were read using an ELISA reader (405 nm) after 30 min.
Positive and negative controls were run on each plate. The positive control of
P. vivax PV210 type consisted of two Gly-Asp-Arg-Ala-Asp-Gly-Gln-Pro-Ala repeats (
Arnot et al., 1985;
McCutchan et al., 1985) glutaraldehyde conjugated to BSA. These positive synthetic peptides were also reacted in wells, A12 (40 pg), B12 (20 pg), C12 (10 pg), D12 (5 pg), and E12 (2.5 pg). The positive control of
P. vivax Pv-247 type was consisted of three Ala-Asn-Gly-Ala-Gly-Asn-Gln-Pro-Gly repeat (
Wirtz et al., 1992) glutaraldehyde conjugated to boiled Casein. These positive synthetic peptides were also reacted in wells, A12 (2 ng), B12 (1 ng), C12 (0.5 ng), D12 (0.25 ng), and E12 (0.125 ng). Uninfected negative control mosquitoes consisted of field collected male
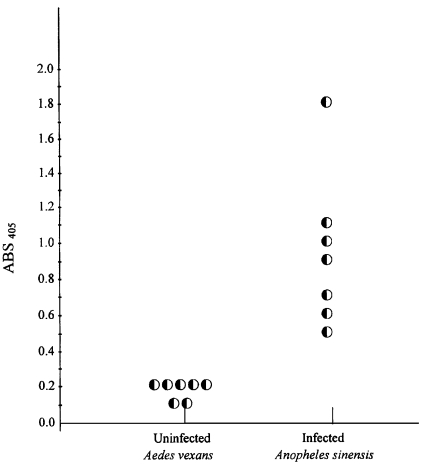
Aedes vexans. Mosquitoes were considered infected if ELISA absorbance values (at 30 min) exceeded twice the mean of four negative controls on the same plate.
RESULTS
Proportion of Anopheline mosquitoes
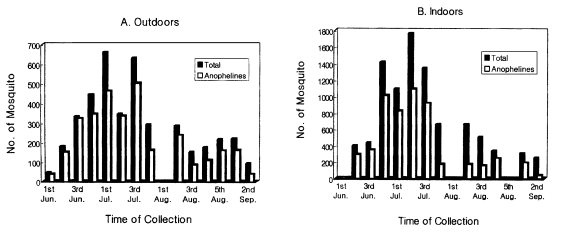
We captured mosquitoes once a week in three different places during the malaria transmission season in Korea. In this survey, total 13,296 mosquitoes were collected from both indoors and outdoors, and 8,650 (65.1%) mosquitoes were found to be of Anopheline mosquitoes. Among the Anopheline mosquitoes, 3,119 (37.0%) Anopheline mosquitoes were captured from outdoors and 5,531 (63.9%) were from indoors. The population density in the indoor collection was shown to be the highest in the second week of July. On the while, the population density in the outdoor collection peaked in the first and the third week of July (
Fig. 2).
We identified 4 different species from possibly 7 species of Anopheline existing in Korea. The identified species included
An. sinensis,
An. yatsushiroensis,
An. lesteri, and
An. koreicus. However, most of them were found to be
An. sinensis (96.4%) while
An. yatsushiroensis accounted for 3.5% of the total captured mosquitoes (
Table 1).
We analyzed 7,820 out of 8,650 captured Anopheline mosquitoes with ELISA. Total 7 Anopheline mosquitoes showed positive for ELISA (
Fig. 3). Therefore, overall positive rate was 0.09 % (7/7,820). The positive rate of 0.14% (5/3,500) was found in Josan-ri and 0.15% (2/1,370) in Jangpa-ri. We did not detect any positive mosquitoes in Dongjung-ri (0/2,950). Sporozoites infected mosquitoes were detected on June 28
th for the first time in Josan-ri (n=2), next on July 5
th in Jangpa-ri (n=l), July 19
th in Josan-ri (n=l), August 9
th in Josan-ri (n=l), September 6
th in Josan-ri (n=l), and the last one on September 13
th in Jangpa-ri (n=l). They were all
An. sinensis. Another 3 species did not react with any kind of monoclonal antibodies (mAbs). The proportion of infected specimens collected from both indoors and outdoors was not significantly different. Three of seven positive mosquitoes (42.9%) were captured indoors and the rest of them (n=4, 57.1%) outdoors. Only one each positive mosquito was captured between 20:00 and 21:00, and 24:00 and 01:00, two positive mosquitoes between 23:00 and 24:00. And three positive mosquitoes were captured between 22:00 and 23:00 (
Table 2).
The types of infected parasites were P. vivax PV210 and PV247. Three of the five mosquitoes caught in Josan-ri reacted to Pv210 type monoclonal antibody (mAb) while others reacted to Pv247 type mAb. In Jangpa-ri mosquito collection, one of the two captured mosquitoes reacted to Pv210 mAb, while the other reacted to PV247 type mAb.
DISCUSSION
Ree et al. (
1967) first discovered the sporozoites from one of 7,517
An. sinensis in Korea. It was also confirmed by polymerase chain reaction (PCR) that
An. sinensis played an important role in a recent malaria transmission in Korea (
Lee et al., 2000). As for
An. yatsushiroensis, one out of 1,469 mosquitoes dissected was infected with oocysts of
P. vivax. In this case,
An. yatsushiroensis was fed directly from a malaria patient's blood (
Hong, 1977). However, few studies have been carried out with the population densities of
P. vivax sporozoites infected mosquitoes. Therefore, we tried to determine the population density of mosquitoes infected with sporozoites in highly malaria epidemic areas in Korea using ELISA method.
Recently, the positive rate of sporozoite-infected mosqutioes was shown to be relatively higher than the result obtained by Ree et al. (
1967). It was 0.09% (7/7,820) in 1999 whereas in 0.013% in 1967 (1/7,517). It is, however, of interest to note that the diagnostic method was different between these two studies. Similar studies had been conducted from 1992 to 1993 by Rattannarithikul et al. (
1996) in Thailand. They found 21
P. falciparum and 7
P. vivax infected mosquitoes out of 7,938 specimens. The positive rate of
P. vivax infected mosquitoes was 0.09%, even though overall positive rate of both parasites was 0.4% total.
The first two positive mosquitoes were detected on June 28
th which was 21 days after the starting day of mosquito collection. The last one was detected on September 13
th. The dates correspond closely to the occurrences of malaria patients in Korea (
Lee et al., 1998). Surprisingly, no malaria patient was reported in Josan-ri in 1999, although the positive rate of
P. vivax sporozoites infected mosquitoes was 0.14% (5/3,500) in the same area. On the other hand, 11 malaria patients in Dongjung-ri were reported in that year, while we were not able to detect positive mosquitoes (n=2,950). In Jangpa-ri, 14 malaria patients were reported and the positive rate of parasites infected mosquitoes was 0.15% (2/1,370). In the late August of that year, we also investigated the anti-circumsporozoite protein (CSP) antibodies of inhabitants in these areas using ELISA with synthetic CSP peptides, and the result showed that the positive rate of ELISA was 3.97% (5/136) in Josan-ri, 15.63% (50/320) in Jangpa-ri, and 22.14% (29/131) in Dongjung-ri (unpublished data), respectively. The relationship between the positive rate of
P. vivax sporozoites infected mosquitoes and the number of patients did not always coincide with each other. The habits of inhabitants who live in or near the epidemic areas should be considered in order to estimate the number of malaria patients or the patterns of the prevalence of malaria. Occurrence of malaria patients might be depends on the chances of exposure to the sporozoite-infected mosquitoes rather than on the population densities of infected mosquitoes in certain areas.
All ELISA positive reactions (n=7) were classified as
An. sinensis. Lee et al. (
2000) also obtained the similar result on identification of vector mosquitoes that were capable of transmitting malaria.
An. sinensis was found to be important as malaria vector as it once was in the past. Recently, it was found that the mean number of sporozoites in
An. sinensis was 818 (n=8; range of 648-1,056) (
Lee et al., 2001), however, we were not able to detect positive
An. yatsushiroensis (n=275), which is considered to be the secondary malaria transmission vector in Korea. Lee et al. (
2000) also failed to detect
P. vivax sporozoites infected mosquitoes in 561
An. yatsushiroensis. Therefore, further studies are in need to confirm the vectorial capacity of
An. yatsushiroensis.
Although we found three of seven positive mosquitoes infected with
P. vivax PV247 type. However, it is presently known that only PV210 type of
P. vivax exists in Korea (
Kho et al., 1999;
Lee et al., 1999). Therefore, some efforts are needed to find a Pv247 type infected malaria patients in Korea.
Notes
-
This study was supported by a grant from the Korea Health 21 R & D Project, Ministry of Health and Welfare, Republic of Korea (HMP-99-P-0018).
ACKNOWLEDGMENTS
We express our gratitude to Dr. R.A. Wirtz, CDC, USA, for supplying the monoclonal antibodies and technical supports.
References
- 1. Arnot DE, Barnwell JW, Tam JP, Nussenzweig V, Nussenzweig RS, Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science 1985;230:815-818.
- 2. Chai IH, Lim GI, Yoon SN, Oh WI, Kim SJ, Chai JY. Occurrence of tertian malaria in a male patient who had never been abroad. Korean J Parasitol 1994;32:195-200.
- 3. Cho SY, Kong Y, Park SM, et al. Two vivax malaria cases detected in Korea. Korean J Parasitol 1994;32:281-284.
- 4. Hong HK. Ecological studies of important mosquitoes in Korea. 1977, Dong Kook University Graduates School; pp 1-55 Ph.D. Theses.
- 5. Kho WG, Park YH, Chung JY, et al. Two new genotypes of Plasmodium vivax circumsporozoite protein found in the Republic of Korea. Korean J Parasitol 1999;37:265-270.
- 6. Lee JS, Kho WG, Lee HW, Seo M, Lee WJ. Current status of vivax malaria among civilians in Korea. Korean J Parasitol 1998;36:241-248.
- 7. Lee JS, Lee WJ, Kim TS, et al. The studies on the geographical distribution of the circumsporozoite protein gene from Korean isolates. The Report of National Institute of Health in Korea 1999;36:150-161.
- 8. Lee HW, Cho SH, Shin EH, et al. Experimental infection of Anopheles sinensis with Korean isolates of Plasmodium vivax. Korean J Parasitol 2001;39:177-183.
- 9. Lee WJ, Lee HW, Shin EH, et al. Vector determination of tertian malaria (Plasmodium vivax) by polymerase chain reaction. Korean J Entomol 2000;30:77-83.
- 10. MacDonald G. The analysis of the sporozoite rate. Trop Dis Bull 1952;49:569-586.
- 11. McCutchan TF, Lai AA, de la Cruz VF, et al. Sequence of the immunodominant epitope for the surface protein on sporozoites of Plasmodium vivax. Science 1985;230:1381-1383.
- 12. Ministry of Health and Social Affairs, Korea. Malaria pre-eradication programme in Korea. Progress Report by National Malaria Eradication Service, 1961-1965. 1966, pp 44-70.
- 13. Paik YH, Ree HI, Shim JC. Malaria in Korea. Jpn J Exp Med 1988;58:55-66.
- 14. Rattanarithikul R, Konishi E, Linthicum KJ. Detection of Plasmodium vivax and Plasmodium falciparum circumsporozoite antigen in anopheline mosquitoes collected in southern Thailand. Am J Trop Med Hyg 1996;54:114-121.
- 15. Ree HI, Hong HK, Paik YH. Study on natural infection of Plasmodium vivax in Anopheles sinensis in Korea. Korean J Parasitol 1967;5:3-4.
- 16. Soh CT, Lee KT, Im KI, et al. Current status of malaria in Korea. Yonsei Rep Trop Med 1985;16:11-18.
- 17. Wirtz RA, Sttabongkot J, Hall T, Burkot TR, Rosenberg R. Development and evaluation of an enzyme-linked immuno-sorbent assay for Plasmodium vivax-VK247 sporozoites. J Med Entomol 1992;29:854-857.
Fig. 1Map of areas studied, see
Table 1. DPRK, Democratic People's Republic of Korea; ROK, Republic of Korea; DMZ, Demilitarized Zone; 1, Josan-ri; 2, Jangpa-ri; 3, Dongjung-ri.

Fig. 2The population densities of mosquitoes collected outdoors (A) and indoors (B).

Fig. 3Detection of Plasmodium vivax sporozoites in individual mosquitoes. (Ordinate = absorbance at 405 nm).

Table 1.The number of Anopheline mosquitoes analyzed by ELISA
Table 1.
|
Areas |
Species
|
Outdoors
|
Indoors
|
|
Total |
As |
Ay |
Al |
Ak |
Total |
As |
Ay |
Al |
Ak |
|
Josan-ri |
1,313 |
1,177 |
134 |
2 |
- |
2,187 |
2,116 |
68 |
2 |
1 |
|
Jangpa-ri |
562 |
549 |
13 |
- |
- |
808 |
796 |
12 |
- |
- |
|
Dongjung-ri |
1,244 |
1,237 |
7 |
- |
- |
1,706 |
1,665 |
41 |
- |
- |
|
Total |
3,119 |
2,963 |
154 |
2 |
- |
4,701 |
4,577 |
121 |
2 |
1 |
Table 2.Sporozoite-infected mosquitoes and subtypes of Plasmodium vivax sporozoites
Table 2.
|
No |
Captured areas |
Collection method |
Species |
Collection day |
Collection time |
Subtype of P. vivax
|
|
1 |
Josan-ri |
Indoor |
An. sinensis
|
28th June |
22:00-23:00 |
Pv247 |
|
2 |
Josan-ri |
Indoor |
An. sinensis
|
28th June |
22:00-23:00 |
Pv247 |
|
3 |
Jangpa-ri |
Outdoor |
An. sinensis
|
5th July |
23:00-24:00 |
Pv210 |
|
4 |
Josan-ri |
Indoor |
An. sinensis
|
19th July |
24:00-01:00 |
Pv210 |
|
5 |
Josan-ri |
Outdoor |
An. sinensis
|
9th Aug. |
22:00-23:00 |
Pv210 |
|
6 |
Josan-ri |
Outdoor |
An. sinensis
|
6th Sep. |
20:00-21:00 |
Pv210 |
|
7 |
Jangpa-ri |
Outdoor |
An. sinensis
|
13th Sep. |
23:00-24:00 |
Pv247 |