Abstract
Studies on Gymnophalloides seoi (Digenea: Gymnophallidae) and human infections are briefly reviewed. This minute intestinal fluke was first discovered from a Korean woman suffering from acute pancreatitis and gastrointestinal troubles. It was described as a new species by Lee, Chai and Hong in 1993. The southwestern coastal village where the patient resided was found to be a highly endemic area, and additional endemic areas have been identified. The parasite is very small, 0.33-0.50 mm long and 0.23-0.33 mm wide, and characterized by the presence of a ventral pit. The first intermediate host remains unknown, but the second intermediate host has been found to be the oyster Crassostrea gigas. Man and the Palearctic oystercatcher Haematopus ostralegus have been shown to be natural definitive hosts, and wading birds including the Kentish plover Charadrius alexandrinus are highly susceptible to experimental infection. Gerbils, hamsters, cats, and several strains of mice were also susceptible laboratory hosts. In experimentally infected mice, the parasites inhabit the small intestine, pinching and sucking the root of villi with their large oral suckers, but they did not invade beyond the mucosa in immunocompetent mice. However, they were found to invade the submucosa in immunosuppressed mice. Human G. seoi infections have been found in at least 25 localities; 23 islands on the Yellow Sea or the South Sea, and 2 western coastal villages. The highest prevalence was found in a village on Aphaedo, Shinan-gun (49% egg positive rate); other areas showed 0.8-25.3% prevalence. Infected people complained of variable degrees of gastrointestinal troubles and indigestion. The infection can be diagnosed by recovery of eggs in the feces; however, an expert is needed to identify the eggs. Praziquantel, 10 mg/kg in single dose, is effective for treatment of human infections. Eating raw oysters in endemic areas should be avoided.
-
Key words: Gymnophalloides seoi, small intestine, histopathology, in situ postures of trematodes, villous atrophy, crypt hyperplasia, immunosuppressed mice
HISTORICAL BACKGROUNDS
The history of Gymnophalloides seoi Lee, Chai and Hong, 1993 (Digenea: Gymnophallidae) started when a woman suffering from acute abdominal discomfort was admitted to the Seoul National University Hospital in 1988. She was diagnosed as acute pancreatitis and was scheduled for surgical intervention (pancreatectomy) to cope with the severe symptoms. At fecal examination, however, very small (0.020-0.025×0.011-0.015 mm) and peculiar trematode eggs were found. Praziquantel 10 mg/kg in single dose was given, and purged with magnesium salts. A thousand small trematodes were recovered from the diarrheic stools. As the symptoms of the patient completely subsided in a few days, the surgery was deferred. The patient was discharged without further clinical problems.
At the time when the adult flukes were recovered, they could not be identified immediately. Review of the available literature suggested that they were a species in the Gymnophallidae, but no pre-existing species was compatible with them. Several specimens were sent to Dr. Hilda Ching (formerly at Hydra Enterprises Ltd., Vancouver, Canada), a specialist for the taxonomy of gymnophallid trematodes. She suggested that our specimens could be the adult stage of
Gymnophalloides tokiensis (
Fujita, 1925), the type species of the genus reported from the Tokyo Bay, Japan. However, because
G. tokiensis was known only by its metacercarial stage, it was unreasonable to synonymize the two without experimental proof. After discussion with her, we came to a conclusion that our specimens could be reported as a new species of
Gymnophalloides (
Lee et al., 1993).
In the mean time, the type specimens of
Gymnophallus macrostoma, which was reported from a Korean bird (
Yamaguti, 1939) and stored in Meguro Parasitological Museum (Tokyo, Japan), were reviewed because it looked morphologically similar to the new species. However, they were apparently different from the new species in lacking the ventral pit, a highly characteristic structure of the genus
Gymnophalloides (
Ching, 1972).
In 1989, after the discovery of the first human case, we visited the village where she resided. The village was on a coastal area of Aphaedo, Shinan-gun, Chollanam-do. The fecal specimens were collected from the villagers and a 49% egg positive rate of
G. seoi was found. There were heavy worm burdens of 106-26,373 per individual (
Lee et al., 1994). It was later confirmed that the reinfection cycle has been continued in this village (
Chai et al., 2000). Moreover, 24 villages on other western and southern coastal areas were proved to be endemic areas (
Chai et al., 1997,
1998a,
2001a). Thus
G. seoi has become one of the important intestinal flukes infecting humans in the Republic of Korea.
During 1990-1991, the oyster
Crassostrea gigas endemic in the village of Aphaedo was found to carry the metacercarial stage (unencysted) of the parasite (
Lee et al., 1995a). Subsequently naturally infected oysters have been found in many islands in Shinan-gun (
Lee et al., 1996) and 2 islands in Gunsan-shi (
Sohn et al., 1998).
The natural final hosts other than man were unknown until 1996, when the adult flukes of
G. seoi were recovered from the small intestine of the Palearctic oystercatcher
Haematopus ostralegus, a species of migrating birds, caught near the endemic areas (
Ryang et al., 2000). Three other avian species, the Kentish plover
Charadrius alexandrinus, Mongolian plover
C. mongolus, and grey plover
Pluvialis squatarola, were found highly susceptible for experimental infection with
G. seoi (
Ryang et al., 2001). In addition, mammals including gerbils, hamsters, cats, and mice (several strains) appeared to be susceptible to experimental infection (
Lee et al., 1997). Among the strains of mice, C3H/HeN mice were the best for growth and development of the flukes (
Chai et al., 1999). The metacercariae were successfully grown into adults using NCTC 109 medium in vitro (
Kook et al., 1997).
Meanwhile, the ultrastructure of
G. seoi metacercariae and adults was studied using scanning (
Choi et al., 1995) and transmission electron microscopes (
Seo et al., 1995), and cysteine proteases were purified from metacercariae (
Choi et al., 1998a) and adults (
Choi et al., 1998b), and their functions were characterized. The histopathological changes were studied in the small intestine of
G. seoi-infected C3H/HeN and C57BL/6 mice, and the effects of immunosuppression were observed (
Chai et al., 2001b).
A great deal of information has been accumulated since G. seoi was first discovered in 1988. The aim of the present study is to review the knowledge obtained during the past 12 years, on the morphology and biology of G. seoi, host-parasite relationships, and human infections.
TAXONOMY AND MORPHOLOGY
Brief descriptive characters of Gymnophallidae Morozov, 1955 (Ching, 1995a)
In order to provide understandings on the taxonomy and morphology of
G. seoi, the descriptive characters of the family Gymnophallidae (
Ching, 1995a) are presented.
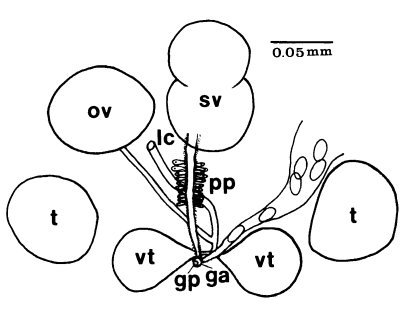
Gymnophallidae: Body small to minute, oval to pyriform. Tegument spined with simple, serrated, or sharply ridged spines within pits. Oral sucker large, sometimes twice the size of ventral sucker, with or without lateral papillae. Sensory papillae arranged in a pattern on oral sucker. Ventral sucker located in middle third of body, with sensory papillae. Pharynx near oral sucker. Esophagus very short. Intestine bifurcate, ceca widely divergent, often enlarged, short, not reaching to midbody, with or without dorsal extensions. Ventral pit present or absent, anterior to ventral sucker. Testes two, round or ovoid, symmetricallly or obliquely arranged to each other, located posterolateral to ventral sucker. Seminal vesicle undivided, bi- or tri-partite. Pars prostatica well developed with tubular or oval vesicle, or absent (in this case gland cells surround genital atrium). Cirrus and cirrus sac absent. Ejaculatory duct and metraterm join to form gonoduct. Genital atrium tubular, or transversely oval. Genital pore median and postbifurcal, immediately anterior, on the edge, or some distance anterior to ventral sucker, never in hindbody. Genital pore wide and pit-like or small, inconspicuous with or without sensory papillae. Ovary rounded or ovoid, anterior to left or right testis, rarely intertesticular. Laurer's canal present. Mehlis gland well developed; seminal receptacle or fertilization chamber present. Vitelline glands varying in shape from paired group of follicles to paired compact to single lobed or compact organ usually located anterior, lateral or directly posterior to ventral sucker. Uterus passing postovarian then anteriorly to cecal bifurcation, in forebody, hindbody, or entire body. Eggs less than 0.04 mm in length, operculate, embryonated when laid. Excretory vesicle V or Y shaped with simple lateral arms; arms extending forward to pharynx. Excretory collecting duct short, with cilia. Flame cells in patterns of two with total of 16 or 24. Excretory pore terminal.
Differential characters of 7 genera of Gymnophallidae
The type genus of Gymnophallidae is
Gymnophallus Odhner, 1900 (type species;
G. deliciosus), and 6 other genera are known so far (
Ching, 1995a). The important differential characters of the 7 genera are listed in
Table 1.
As
Gymnophalloides heardi (
Ching, 1995b) has been added as a new member, the generic characteristics of
Gymnophalloides (
Lee et al., 1993) is redescribed as follows.
Gymnophalloides: Body very small, oval to pyriform, spinose, with more or less muscular ventral pit located near the level of pharynx or postcecal, anterior to ventral sucker. Oral sucker subterminal, large, with prominent lateral projections on each side; usually twice as large as ventral sucker. Ventral sucker in posterior fourth to fifth of body. Prepharynx short to absent; pharynx present; esophagus short. Ceca saccate, dilated, very short, never passing midbody. Testes paired, symmetrical, in posterior half of body; seminal vesicle pretesticular, voluminous, frequently bipartite; pars prostatica well developed, posteroventral to seminal vesicle. Genital atrium shallow. Genital pore median, small immediately anterior to ventral sucker, nonmuscular. Ovary ovoid, dextral, pretesticular. Vitellarium a single, thickly lobed organ, or vitellaria paired, compact masses, close to ventral sucker. Uterus filling available space in anterior and middle thirds of body. Eggs small, elliptical, thin-shelled. Excretory vesicle U or V shaped, arms long. Intestinal parasites of humans, rodents, and birds.
Type species: Gymnophalloides tokiensis Fujita, 1925 (based on metacercaria).
Description of Gymnophalloides seoi Lee, Chai and Hong, 1993
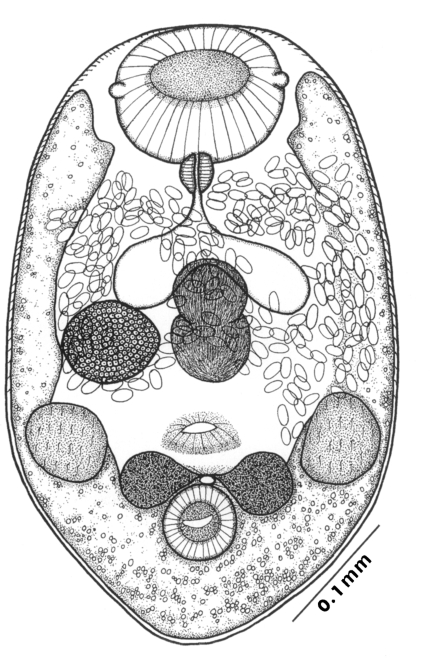
The morphological characters of
G. seoi were described by Lee et al. (
1993) for adult flukes and eggs, and by Lee et al. (
1995a) for metacercariae. The descriptions are based on 10 whole mounts, and given in micrometers.
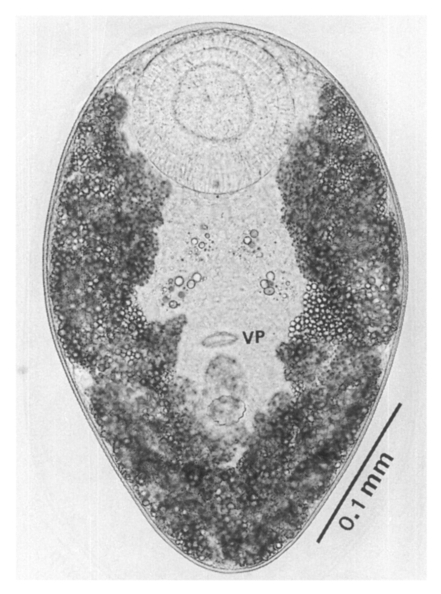
Adults (Figs. 1-4): Body broadly oval, rounded anteriorly, slightly pointed posteriorly, 325-500 (416) long, 225-325 (275) wide at midbody. Anterior 2/3 of body covered with fine tegumental spines. Oral sucker subterminal, large and muscular, 95-155 (120) in transverse diameter, with prominent lateral projections on each side. Prepharynx not seen. Pharynx well developed, muscular, 15-35 (24) by 20-40 (34). Esophagus short. Ceca short, saccate, and inflated, usually ending before midbody. Ventral pit median, transversely elongated, surrounded by strong muscle fibers, 21-32 (25) wide, at about 1/3 of body length from posterior end, 18-35 (27) anterior to ventral sucker. Ventral sucker round, 50-70 (57) in diameter, at 1/4-1/5 of body length from posterior end; sucker width ratio 1:0.419-0.579. Testes 2, ovoid, symmetrical, lateral at level of ventral pit and ventral sucker; right testis 40-130 (83) by 20-60 (39); left testis 55-100 (78) by 25-50 (37). Seminal vesicle frequently bipartite, 50-90 (60) by 35-100 (70), between ceca and ventral pit. Pars prostatica well developed, posteroventral to seminal vesicle. Genital atrium shallow. Genital pore small, inconspicuous, opening at level of anterior margin of ventral sucker, not surrounded by muscle fibers. Ovary oval, 30-90 (64) by 25-90 (48), anteromedial to right testis. Laurer's canal present, opening dorsally. Vitellaria 2 compact masses, rarely lobed, anterolateral to ventral sucker, right 30-50 (40), left 30-55 (41) in diameter. Uterus with anterior loops to level of pharynx, mostly in middle 1/3 of body. Uterine eggs numerous (see Eggs for details). Excretory bladder V-shaped, arms reaching to oral sucker.
Type host: Homo sapiens.
Other host: Palearctic oystercatcher Haematopus ostralegus.
Location: Gastrointestinal tract.
Type locality: Shinan-gun, Jeonranam-do, Korea.
Specimens deposited: Holotype, SNU (Seoul National University) Helm. Coll. no. 9225, 9228-9324: paratypes, USNM Helm. Coll. (Beltsville) no. 82478, Meguro Parasitological Museum (Tokyo) no. 19568.
Etymology: The specific name is in honor of the late Prof. Byong-Seol Seo, Department of Parasitology, Seoul National University College of Medicine, who dedicated his life for studies on parasitology in Korea.
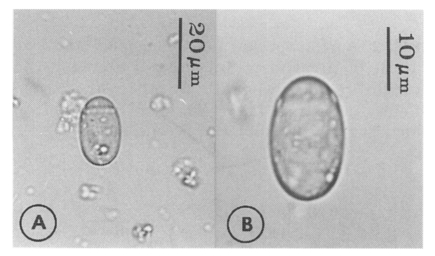
Eggs (Fig. 5): Small, operculate, elliptical, 20-25 (21) by 11-15 (13), with thin, transparent shell. Concentration techniques are recommended to detect eggs in the human or animal feces. On Kato-Katz thick smears, they are distinguished with difficulty from air bubbles or other artifacts.
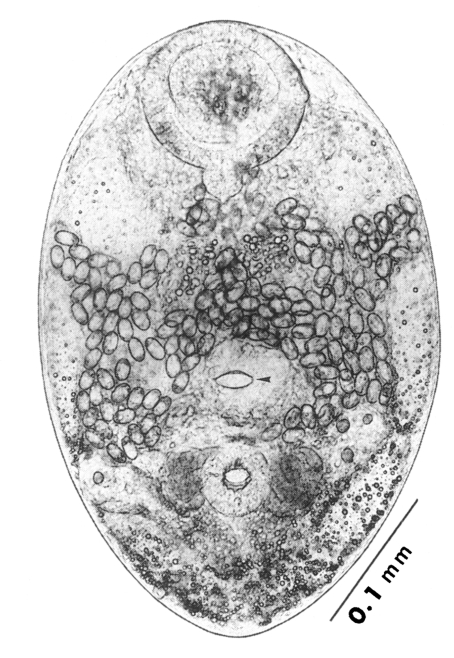
Metacercariae (Fig. 6): Body small and pyriform, and covered entirely with fine tegumental spines. Anterior end round, posterior end slightly pointed. The body length 310-386 (346), width 205-258 (228). Oral sucker large and well developed, 94-127 (109) by 102-140 (122), with two ventrolateral lips. Pharynx round and muscular, 28-37 (31) by 31-47 (37). Esophagus very short, and succeeded to inflated oval ceca. Ventral pit located posterior to midline, 7-19 (12) by 24-38 (30). Ventral sucker located about 1/3 of body length from posterior end, 46-66 (52) by 51-67 (56). Genital pore small, inconspicuous, maximum width one half the size of ventral pit, opening at anterior margin of ventral sucker, not surrounded by prominent muscle fibers. Ovary and testes in posterior 1/3, but not easily observed due to numerous excretory granules. Excretory bladder V shaped extending to the level of oral sucker, filled with many tiny refractile granules.
Host: Crassostrea gigas (oyster).
Location: Mantle.
Locality: Shinan-gun, Jeonranam-do, Korea.
Specimens deposited: Holotype, SNU (Seoul National University) Helm. Coll. no. 9501: paratypes, SNU Hem. Coll. No. 9502-9550.
Taxonomic significance of G. seoi
Among the 7 genera of Gymnophallidae Morozov, 1955, listed by Ching (
1972,
1995a) and Schell (
1985), only
Gymnophalloides Fujita, 1925 and
Lacunovermis Ching, 1965 include species that have a ventral pit. Yamaguti (
1971) synonymized the two genera on the basis of the presence of a ventral pit in both genera. However, Ching (
1972) examined the metacercarial specimens of
G. tokiensis from oysters from Japan, and reported that the two genera are distinguished by the size, location, and musculature of the genital pore. Whereas
Gymnophalloides has a small, inconspicuous and nonmuscular genital pore,
Lacunovermis has a wide and muscular genital pore comparable to the ventral pit (
Ching, 1972). It was also noted that the genital pore of
Lacunovermis is at some distance anterior to the ventral sucker, whereas that of
Gymnophalloides opens on the anterior margin of the ventral sucker (
Ching, 1972).
Gymnophalloides seoi closely resembles
Gymnophalloides tokiensis Fujita, 1925 (known only as a metacercaria), redescribed by Ching (
1972). The only recognizable difference between the two species is the shape, position and orientation of the seminal vesicle in metacercariae (
Lee et al., 1995a). Comparison of adult flukes is not possible at present, because
G. tokiensis adults have never been found, even from the Tokyo Bay, Japan, where its metacercariae were first discovered (
Fujita, 1925). In
G. tokiensis metacercariae, the seminal vesicle is bipartite and located between the ventral pit and ventral sucker, and its posterior portion is curved dorsally (
Ching, 1972). In contrast, in
G. seoi metacercariae, it is in most cases mono-sac and situated more anteriorly between the ceca and ventral pit, and not curved dorsally (
Lee et al., 1995a).
Gymnophalloides heardi (
Ching, 1995b) was reported as a new species after
G. seoi. The most remarkable difference of the two is the number and shape of vitellaria (
Ching, 1995b); in
G. heardi, the vitellarium is only one in number and thickly lobed, but in
G. seoi, vitellaria are two compact masses, rarely lobed. The ventral pit of
G. heardi is located near the level of the pharynx (
Ching, 1995b), but that of
G. seoi is located more posteriorly; postcecal and posterior to the seminal vesicle (
Lee et al., 1993). Other differential characters of
G. seoi include its larger body size, and more strongly muscular ventral pit. Moreover,
G. seoi takes man and wild birds as natural definitive hosts (
G. heardi; rice rats), and oysters (a bivalve) as the intermediate host (
G. heardi; a pulmonate gastropod).
Gymnophallus macrostoma Yamaguti, 1939, reported from a Korean bird (Meguro Parasitological Museum, Tokyo, Japan) is different from
G. seoi in having no ventral pit, no pair of ventrolateral muscular projections on the oral sucker, ovary anterior to midbody, large vitellaria, and narrower body (
Lee et al., 1993). Yamaguti (
1939) cautiously mentioned similarity of
G. macrostoma (adult) and
G. tokiensis (metacercaria) especially in the location of the ovary, not realizing that the latter has a ventral pit and the former does not. Stunkard and Uzmann (
1958) again mentioned the similarity of the two. James (
1964) regarded them synonymous and emended
Gymnophalloides using the description of
G. macrostoma adults. Ching (
1965) did not agree with the emendation of
Gymnophalloides by James (
1964); instead, she used the emended diagnosis for a new genus,
Meiogymnophallus, which included
M. macrostoma (
Yamaguti, 1939) and related species lacking a ventral pit.
ULTRASTRUCTURE
Surface ultrastructure (Figs. 7-10)
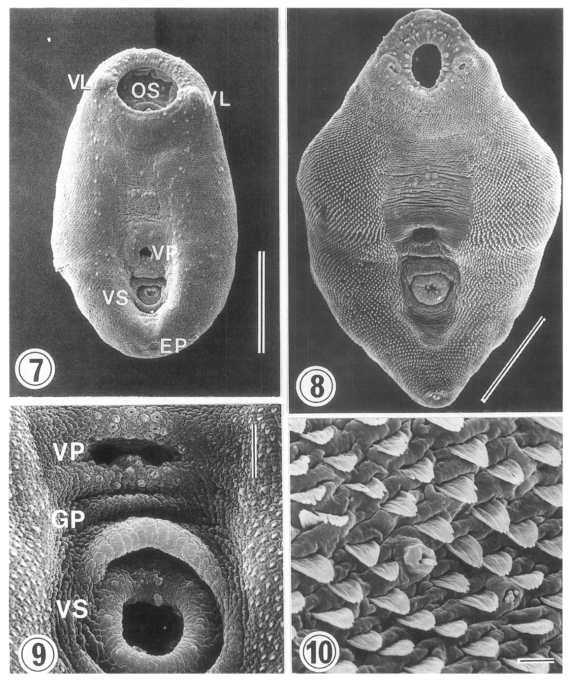
The surface ultrastructure of
G. seoi was studied in metacercariae and adults using a scanning electron microscope (SEM) (
Choi et al., 1995). The metacercariae were isolated from naturally infected oysters
C. gigas, and the adult flukes were harvested from the intestine of experimentally infected C3H mice at day 3 post-infection (PI).
Metacercariae (Figs. 7 & 9): Ovoid or pyriform, slightly curved ventrally, not encysted (
Fig. 7). Oral sucker 2-3 times larger than ventral sucker. Ventral sucker posterior to ventral pit at the level of posterior third of body. Uniciliated sensory papillae (type I) arranged bilaterally in a row throughout the entire body length. Tegument of the lip of oral sucker wrinkled radially, and devoid of tegumental spines. The lip of oral sucker has two kinds of type I papillae, small and large ones, arranged circularly around the sucker; large type I papillae form a circle along the lip of sucker, while small type I papillae mainly distribute at the dorsal side of the lip. Single type I papillae seen bilaterally at inner side of oral sucker. On each side of oral sucker locate lateral projections (= ventrolateral lips), elevated-dome shape and covered with 3-5 digitated tegumental spines. Many type I papillae distributed around and on ventrolateral lips. Surface below oral sucker armed densely with 3-5 digitated tegumental spines. Lumen of ventral pit circular or transverse slit shape and its surface not covered with tegumental spines. Genital pore noticeable with difficulty. Ventral sucker, covered with cobble-stone like cytoplasmic processes, has 6 type I papillae on its lip (
Fig. 9). Excretory pore subterminal and covered sparsely with smaller tegumental spines than those of anterior body.
Dorsal body surface covered with tongueshaped tegumental spines with 4-5 points at anterior portion and poorly developed spines at posterior portion. Sensory papillae scattered on dorsal surface.
Adults (Figs. 8 & 10): Rhomboid or elliptical, covered with tegumental spines except ventromedian area between two suckers. Ventral pit at the level of equatorial line of body. Oral sucker has two kinds of type I papillae (small and large) encircling the lip as seen in metacercariae. Ventrolateral lip retracted slightly and had many type I papillae on its base. Tegumental spines around oral sucker digitated into 5-6 points, cytoplasmic folds irregular, and type I papillae seen between spines. Surface anterior to ventral pit not covered with spines, but distributed two groups of type I papillae, each composed of 5-6 in number. Surface lateral to ventral pit covered with 6-7 digitated spines, larger than those around oral sucker. Transverse cytoplasmic folds well developed and single type I papillae distributed between spines. Except for surface posterior to ventral sucker, areas around ventral pit and ventral sucker devoid of spines. Ventral pit well developed, covered with cobble-stone like cytoplasmic processes, and 12-15 type I papillae distributed densely around its border. Transverse cytoplasmic folds observed at surface between ventral pit and ventral sucker, and type I papillae distributed on cytoplasmic ridges. Genital pore a transverse slit, just anterior to ventral sucker. Surface of ventral sucker devoid of spines. Lip of ventral sucker wrinkled radially, and its lumen very small compared to oral sucker. Type I papillae distributed along the margin of ventral sucker. Tegumental spines digitated into 5-7 points at middle and posterior one-third of body. Excretory pore subterminal.
On dorsal surface, many type I papillae scattered through entire body. Tegumental spines of dorsal surface had 8-10 points in anterior (
Fig. 10), 6-7 ones in middle, and 6-7 ones in posterior third of body. Size of spines biggest in middle third and smallest in posterior third of body. Spine density decrease from anteroir toward posterior portion of body.
It is peculiar that only one type of sensory papillae (uniciliated type I) is seen on the surface of
G. seoi metacercariae and adults. Other trematodes have in general at least two types, i.e., type I and type II, or type III (
Bennett and Threadgold, 1975;
Fujino et al., 1979;
Lee et al., 1984,
1987;
Yu et al., 1994). Instead of being equipped with various types of papillae, however,
G. seoi had two kinds of type I papillae, small and large, encircling the lip of the oral sucker.
In contrast to
G. seoi,
Parvatrema timondavidi, a member of Gymnophallidae, has three types of papillae; type I, type II (round swellings without cilium), and type III (
Yu et al., 1994). In
P. timondavidi, many type I and type II papillae are distributed especially around the lip of the oral sucker. Type III papillae (round swellings with a pit in the center), are located symmetrically on the medial side of lateral projections (ventrolateral lips) in metacercariae, but they disappear in adult flukes, which suggests stage-specific action of the type III papillae (
Yu et al., 1994). The genital pore of
G. seoi is armed with grouped type I papillae, but that of
Parvatrema affinis and
Lacunovermis macomae has type II sensory papillae (
Pekkarinen, 1984,
1987).
Tegumental spines of
G. seoi become larger and more digitated as the fluke matures. On the ventral surface spines are dense over the anterior body but sparse over the posterior body. The shape and the distribution of tegumental spines are different by species of parasites according to their migratory behaviors, migration routes, developmental stages, and final habitat in the host (
Bennett and Threadgold, 1975;
Fujino et al., 1979;
Lee et al., 1984;
Hong et al., 1991). They are more simply pointed in juveniles than in adults, and at migratory stages than at non-migratory ones (
Bennett and Threadgold, 1975;
Lee et al., 1984). The surface of
G. seoi is covered with narrow, pointed spines in metacercariae, but with broad, more pointed spines in adults. The simple spines in metacercarial stage seem to be useful for migration in the body of the intermediate host (oysters), while those in adults seem to be good for anchorage in the body (intestine of man or birds).
The ventral pit is a transverse slit without connection to the internal organs (
Ching, 1972). The ventral pit of
G. seoi is more prominent than the genital pore, and many type I papillae are aggregated around its lumen. In contrast, the ventral pit of
Lacunovermis is a transverse slit with a narrow lumen, sometimes less pronounced than the genital pore, and a sensory papilla is singly present on each side of the pit (
Pekkarinen and Ching, 1994). These differences appear to be useful keys for differentiation of the two species. The exact functions of the ventral pit, however, are unknown, although it was suggested to play the role of a sphincter or an adhesive organ, based on the transmission electron microscopic findings (
Seo et al., 1995).
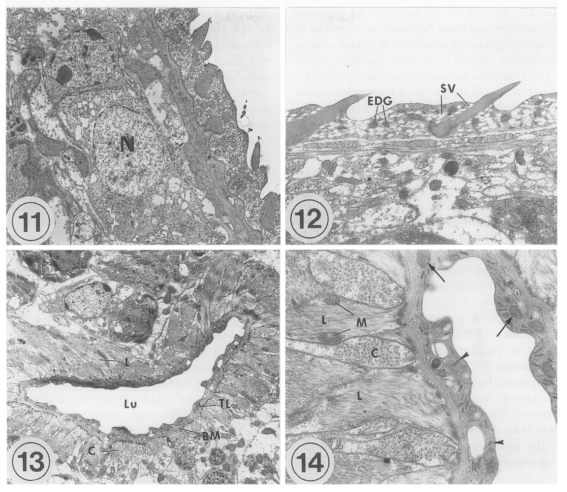
The transmission electron microscopic (TEM) ultrastructure of
G. seoi was studied in metacercariae and adults (
Seo et al., 1995). The metacercariae were isolated from naturally infected oysters
C. gigas, and the adult flukes were harvested from the intestine of experimentally infected C3H mice at day 7 PI.
Metacercariae (Fig. 11): The external surface is enclosed with a syncytial layer. The matrix of the syncytial layer is composed of electron-dense materials. In the matrix, numerous small vacuoles, mitochondria, and tegumental spines are distributed. The outer surface of the tegument is even with few folds, and located tegumental spines at equal intervals. Beneath the internal plasma membrane, an electrolucent basement membrane is located, the breadth of which is approximately 100 nm. Under the basement membrane located circular and longitudinal muscle bundles, and tegumental cells with plenty of lysosomes are present. The average size of the tegumental spines is 0.008 mm in length and 0.005 mm in width.
The oral sucker is composed of the syncytial layer, basement membrane, and muscle layer. The syncytial layer has a lot of vacuoles and small numbers of mitochondria. The thickness of the syncytial layer and basement membrane of the oral sucker is thinner than that of the other tegument in general. There are electrondense granules, which are larger than the vacuoles. Beneath the basement membrane located bundles of circular muscles. Beneath them, longitudinal muscles are well developed. Spindle-shaped muscle cell nuclei are distributed parallel to the muscle fibers. Between the muscle layers, tegumental cells having an electron-dense nucleus are seen. The ultrastructure of the ventral sucker is very similar to that of the oral sucker, except for numerous syncytial processes and folds of the syncytial layer in the ventral sucker. Parenchymal cells with an electron-dense nucleus are present among the circular muscle bundles.
Adults (Figs. 12-14): Tegumental spines are slightly longer than in metacercariae, and intervals between the spines are more uniform. In the oral sucker, the compactness of the muscle layer increases slightly in adults, especially for circular muscles. Otherwise, there are no remarkable ultrastructural differences between metacercariae and adults.
The lumen of the ventral pit is 0.02 mm wide and 0.003 mm long. There are slight elevations of the syncytial layer toward the lumen. The syncytial layer is very thin, 0.0005 mm, the thinnest of the whole tegument of G. seoi. In the syncytial layer, there are few vacuoles unlike the tegumental layer. Glycogen granules, mitochondria, and numerous microtubules are distributed. The basement membrane is relatively thick, and along the basement membrane circular and longitudinal muscle bundles are present. The muscle bundles are well-developed, densely populated, and several mitochondria are observed at the margin of the longitudinal muscles.
Functional aspects of the TEM ultrastructure
According to previous studies, spines are usually present on the tegument of digenean trematodes and their functions are believed to help the parasites attach to the host tissues (
Lumsden, 1975). In
G. seoi, the tegumental spines increase in number as they grow to be adults. It seems to be a consequence of development, and for easier attachment to the host tissues.
G. seoi has a smaller number of foldings and thinner syncytial layer compared with other parasites.
The exact function of small vacuoles (
Fig. 12) in the syncytial layer of
G. seoi is not clear. It seems that they, by any means, participate in absorption and secretion. The digenean tegument plays a vital role for survival of the parasites doing important functions such as absorption of the nutrients, secretion, or discharge of waste products (
Lumsden, 1975). The tegument of aspidogastrids becomes long microtubules as they adapt to the host (
Rohde and Watson, 1992), to live in the narrow ducts of the rectal glands of fish.
G. seoi has short cytoplasmic processes in comparison to other trematodes, suggesting that the attachment of
G. seoi to host tissues might not be firm. Instead, the sucking power of its oral sucker should be very strong to maintain its habitat.
The morphology and the distribution of the tegumental spines vary according to diverse factors including the habitat of parasites, migratory behaviors in the host, and developmental status (
Lumsden, 1975). In the case of parasites migrating through the host tissues such as
Paragonimus westermani or
Fasciola hepatica, their spines are transformed into a different form, that is suitable for migration. Such spines are more subdivided as they reach the normal habitat (
Bennett, 1975). Although lacking a migration phase,
Clonorchis sinensis which enter the bile duct through the ampulla of Vater retain spines during their larval stage, and become adults when they lose the spines (
Fujino et al., 1979). The digenean tegument secretes enzymes such as phosphatases, nonspecific esterases, or aminopeptidases (
Erasmus and Ohman, 1963,
1965). Thus the granules observed in the sucker may help
G. seoi digest host cells by secreting enzymes or other chemical substances.
In G. seoi, the oral sucker is surrounded with big muscle bundles, for easier attachment to the villi of the host intestine. It is also surrounded with the syncytial layer and a thin basement membrane. In the syncytial layer, numerous vacuoles and mitochondria are present, and glycogen granules are found. The syncytial layer has few folds, but has well-developed muscle layers underneath. The ventral sucker has more folds in the syncytial layer than the oral sucker, but the development of the muscle layer on the ventral sucker is poor.
The ventral pit of
G. seoi is encircled with a thin syncytial layer. But the syncytial layer of the ventral pit has a different structure in comparison to the tegument or suckers. Instead of numerous granules and folds seen in the tegumental layer or suckers, many mitochondria and microtubules are found throughout the syncytial layer of the ventral pit. A number of big circular and longitudinal muscle bundles are distributed below the syncytial layer, and the muscle bundles have mitochondria. In this regard, the ventral pit is suggested to be a sphincter or an accessory adhesive organ (
Seo et al., 1995).
LIFE HISTORY
Second intermediate host
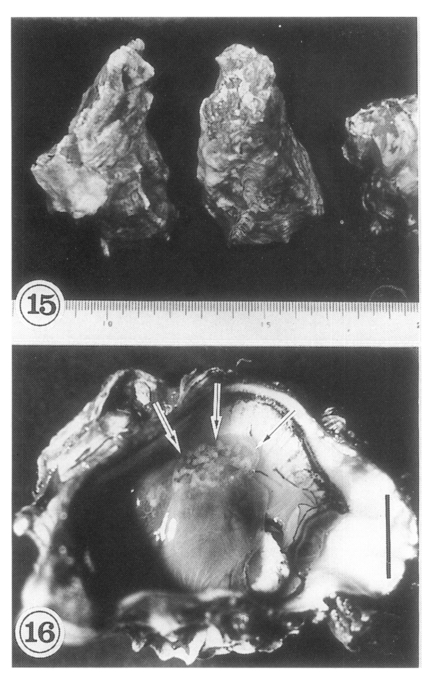
The oysters
Crassostrea gigas (
Fig. 15) collected in Shinan-gun were found infected with the metacercariae of
G. seoi (
Lee et al., 1995a). Oysters from many other areas were also found carrying the metacercariae (
Lee et al., 1996). In Shinan-gun, the average number of metacercariae found was 610 (range 2-4,792) per oyster (
Table 2;
Lee et al., 1995a). The metacercariae are mainly located on the mantle surface near the hinge of the shell, and as the infection density increases they spread toward the mouth of the oyster. Heavily infected regions appear as whitish dots (
Fig. 16), and are easily seen with the naked eye. The shell side of the infected oyster reveals brownish discolorations.
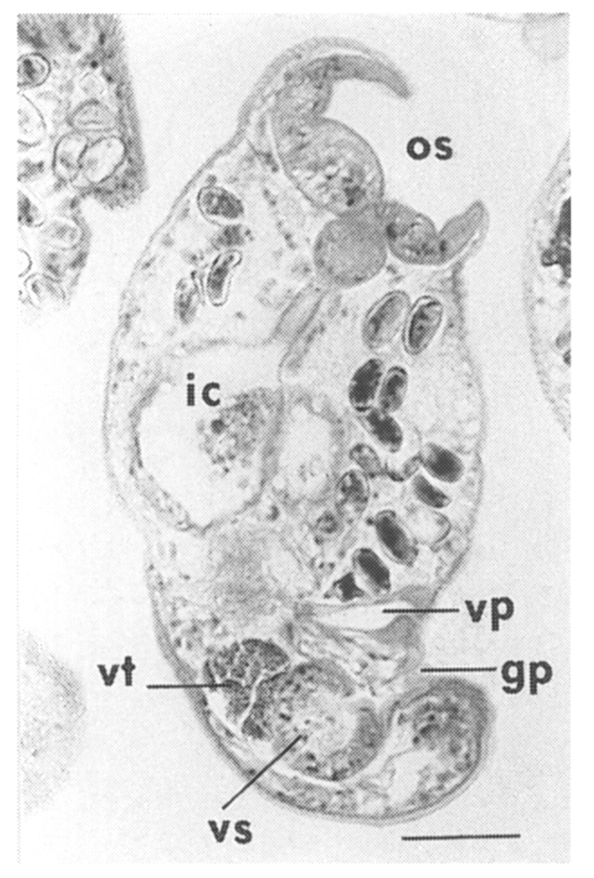
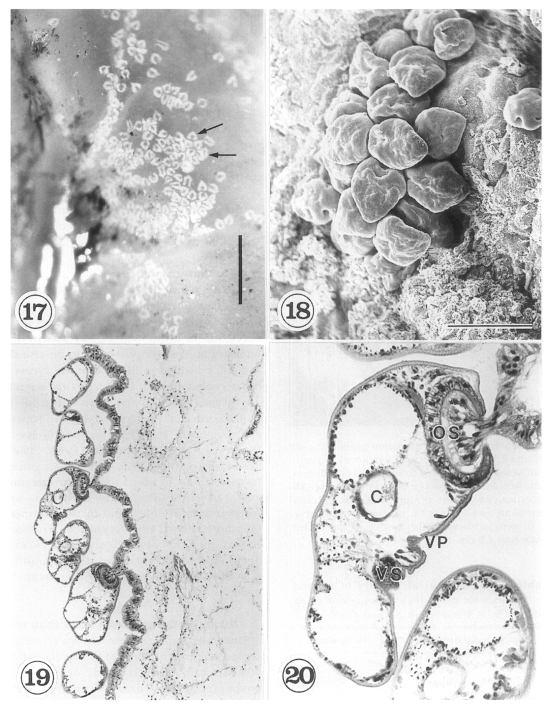
The metacercariae are attached to the extrapallial epithelia of the mantle of oysters (
Figs. 17-20) with their large and well developed oral sucker. They are frequently found in groups (
Figs. 17-19). In midline sections of the metacercariae, the oral sucker, short blind ceca, ventral sucker, genital pore, and ventral pit may be seen (
Fig. 20).
Metacercariae of
Parvatrema timondavidi were discovered from
Tapes philippinarum, a species of marine clam, in the Republic of Korea (
Yu et al., 1993). In addition, metacercariae of
Meiogymnophallus sp. were found in razor clams (unpublished observation). The metacercariae of
G. seoi have never been found in any molluscan species except oysters. There is a possibility that other marine bivalves serve as the second intermediate host and may be sources of human
G. seoi infection; this should be investigated further.
The first intermediate host of
G. seoi has not been determined, therefore, the larval development of the parasite from sporocysts to cercariae is unknown. However, the first intermediate host is presumed to be also the oysters, considering the known life cycles of other gymnophallids. In
Parvatrema borealis, sporocysts and cercariae are found in the molluscan host,
Gemma gemma, and metacercariae in the same host and in polychaetes (
Stunkard, 1962). James (
1960,
1964) described the unique life cycle of
Parvatrema homoeotecnum, in which the primary germinal sac, tailed daughter sac, cercaria, and metacercaria are all found in the gastropod intermediate host,
Littorina saxatilis tenebrosa. Szidat (
1962) also described various developmental stages of
Gymnophallus australis from the same molluscan host, the mussel.
Since the oysters were shown to be the second intermediate host of
G. seoi (
Lee et al., 1995a), wild birds that eat oysters were suspected as natural definitive hosts. Consequently, oystercatchers
Haematopus ostralegus (
Fig. 21), a species of waders, were found to harbor the adult flukes (
Ryang et al., 2000). The prevalence among the oystercatchers was 71.4% (positive in 5 of 7 birds examined). The birds were damaged by gunshot during collection, so not all worms were recovered. However, 302-1,660 (mean 892) adult specimens of
G. seoi were collected from the intestinal tract (
Ryang et al., 2000).
Eleven species of oystercatchers are currently known in the world, but only
H. ostralegus is found in Korea (
Sibly and Monroe, 1990;
Howard and Moore, 1991). They are migratory birds; some breed on uninhabited islands (
Won, 1993), but many found in Korea are temporary visitors or are overwintering (
David and David, 1995). Since they are migratory birds,
G. seoi could be distributed in coastal areas of neighboring countries such as China, Japan, and eastern coasts of Russia, where oysters and oystercatchers are present.
Other species of wild birds may also be natural definitive hosts of
G. seoi. The high susceptibility of 3 species of plovers to experimental
G. seoi infection was confirmed (
Ryang et al., 2001); the Kentish plover
Charadrius alexandrinus, Mongolian plover
C. mongolus, and grey plover
Pluvialis squatarola in decreasing order of susceptibility. These birds are also suspected to be natural definitive hosts.
Mammals including rodents are also suspected as natural definitive hosts for
G. seoi, since mice, rats, hamsters, and gerbils were found susceptible to experimental infection (
Lee et al., 1997). Humans are mammals, and a natural definitive host, with heavy worm burdens (
Lee et al., 1994), which supports the above speculation. It is also supported by the fact that the natural definitive host for
G. heardi is a species of rice rats (
Ching, 1995b).
HOST-PARASITE RELATIONSHIPS
Habitat in the definitive host
The major habitat of
G. seoi in the definitive host is believed to be the small intestine; the duodenum, jejunum, and ileum. It was confirmed in Palearctic oystercatchers, a natural definitive host (
Ryang et al., 2000). In other avian species, the Kentish plover, Mongolian plover, and grey plover, experimentally infected with
G. seoi, the habitat of adult flukes was also confirmed to be the small intestine (
Ryang et al., 2001). In experimental animals including cats, dogs, and rodents such as mice, rats, hamsters, gerbils, and guinea pigs (
Lee et al., 1997), the adult flukes were harvested also from the small intestine.
In the human host, the major habitat of
G. seoi is also thought to be the small intestine. Treatment of patients with praziquantel administration followed by purgation enabled recovery of thousands of adult flukes from the diarrheic stools (
Lee et al., 1993,
1994). However, the first patient of
G. seoi suffered from acute pancreatitis (
Lee et al., 1993), and two other cases accompanied diabetes mellitus (
Lee et al., 1995a). It was thus suspected that
G. seoi could infect the pancreatic duct in humans as well as other animal hosts. Other gymnophallids were found in the bursa Fabricii and gallbladder of shorebirds as well as their intestines (
Yamaguti, 1971;
Schell, 1985). However, in our series of studies, which used rodents as the host (
Lee et al., 1995a,
1997;
Chai et al., 1999,
2001b),
G. seoi have never been found from locations other than the small intestine.
Nevertheless, the possibility should be retained for
G. seoi to be found from extraintestinal locations in the definitive host. Larger experimental animals may be needed to verify the possibility of
G. seoi infecting the pancreatic duct or other locations. Moreover, since the flukes were able to invade the submucosa of immunosuppressed mice (
Chai et al., 2001b), there is a possibility that
G. seoi eggs may be transferred to remote organs in immunocompromised hosts. This should be studied further.
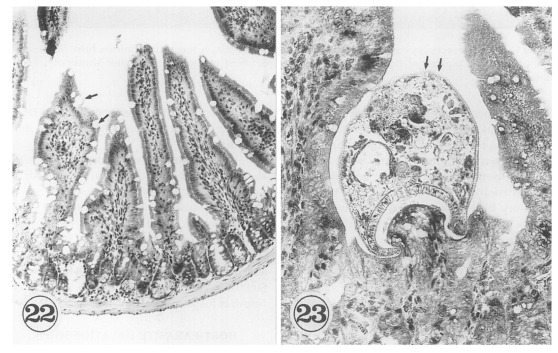
The in situ postures of
G. seoi were shown in the small intestine of immunocompetent and immunosuppressed mice (
Chai et al., 2001b). The purpose of immunosuppression was to enhance the survival of worms in the mouse intestine. The small intestines of
G. seoi-infected immunocompetent mice (C3H/HeN) showed atrophy of the villi and hyperplasia of the crypts, with strong goblet cell hyperplasia along the mucosal epithelial layers (
Fig. 22). Adult flukes of
G. seoi were characteristically seen pinching and sucking the epithelial layer of villi with the oral suckers (
Fig. 23). The appearance rate of flukes in tissue sections, however, was relatively low.
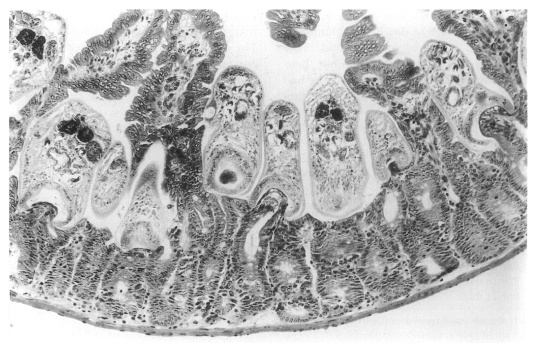
In contrast, in immunosuppressed C57BL/6 mice infected with
G. seoi, numerous adult flukes were found sectioned (
Fig. 24), pinching, sucking, and eating the root of villi in the small intestine (
Chai et al., 2001b). Viewing from the postures of the adult flukes, their in situ behaviors could also be imagined. Most flukes contained fragments of host mucosal tissues within the oral suckers. Eventually displacement as well as complete loss of the villi occurred near groups of worms. A peculiar finding was that a few adult flukes were seen to have penetrated deep into the submucosa of the small intestine, just facing the serosa. These flukes also retained fragments of mucosal tissue within the oral suckers.
The in situ postures of
G. seoi adults (
Chai et al., 2001b) were essentially the same as those observed in their second intermediate host, being attached to the mantle surface of the oysters (
Lee et al., 1995a). Considering the in situ postures, behaviors of the flukes in the mouse intestine are suggested to include vigorous movement and rotation in situ, and giving pressures to the neighboring villi. Thus, it is further suggested that mechanical irritation may be an important mechanism in the pathogenesis of
G. seoi infection. This will be discussed in more details later.
The susceptibility of animal hosts to infection with a parasitic helminth can be assessed by observing the worm recovery after experimental infection (
Chai et al., 1984), and the developmental status and fecundity of worms (
Ito and Kamiyama, 1987). The susceptibility of laboratory animals to
G. seoi infection was studied by observing the worm recovery and maturity of worms especially the number of intrauterine eggs (
Lee et al., 1997). Among 6 species of mammals examined, the highest worm recovery was observed in gerbils (28.0% on average), followed by hamsters (14.2%), and cats (10.9%) at day 7 PI. Rats, dogs, and guinea pigs were less susceptible, with worm recovery at day 7 PI of 0.0-4.0%. Among 5 strains of mice, the worm recovery varied (
Lee et al., 1997); highest in KK mice (12.4%), followed by C3H/HeN (11.8%), ICR (9.6%), BALB/c (6.4%), and ddY mice (6.3%).
The sexual maturity of worms, as judged by the number of intrauterine eggs, was highest in C3H/HeN mice, followed by ddY and ICR mice (
Lee et al., 1997). The worms recovered from C3H/HeN mice at day 7 PI contained as many as 103.7 eggs on average, and those from ddY mice 86.3 eggs per worm, but those recovered from ICR, KK, and BALB/c mice had smaller numbers of uterine eggs. In this connection, C3H/HeN mice were considered a useful animal model for experimental infection with
G. seoi.
There have been many reports on the use of immunosuppressive drugs such as prednisolone to enhance host susceptibility to intestinal helminth infections. In
Metagonimus yokogawai infection, for example, prednisolone-treated mice showed remarkably enhanced worm recovery and significantly longer survival of worms than control mice (
Chai et al., 1984). Similarly, eggs of
Hymenolepis nana inoculated into the rat failed to mature in the intestine except when the host was treated with cortisone from the day of cyst maturation (
Ito, 1983).
Although C3H/HeN mice were regarded as a fairly susceptible host for
G. seoi infection, the worm recovery from these mice at day 7 PI was unsatisfactory (
Lee et al., 1997). Therefore, in order to enhance the worm recovery, C3H/HeN mice were immunosuppressed by injection with prednisolone (
Lee et al., 1997;
Chai et al., 1999). The results were highly successful; remarkably higher worm recovery was obtained in immunosuppressed C3H/HeN mice than in immunocompetent controls (
Table 3). The worm recovery showed a strong correlation with the duration of immunosuppression of the mice (
Lee et al., 1997).
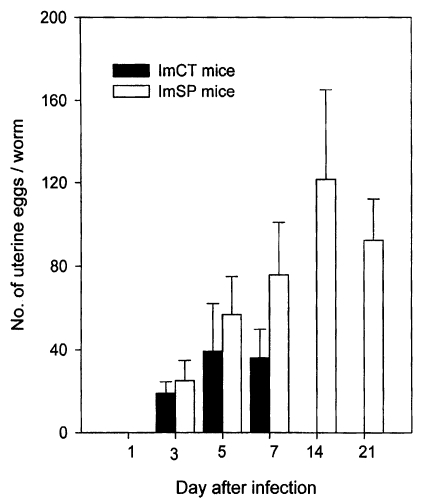
Immunosuppression of C3H/HeN mice also enhanced the fecundity of
G. seoi. Worms recovered from immunosuppressed mice at day 7 PI were all fully mature, containing more than 120 uterine eggs per worm (
Lee et al., 1997). The eggs began to appear in the uteri of 3-day old worms, and the number of eggs quickly increased in accordance with aging of the worms (
Chai et al., 1999). At days 5 and 7 PI, worms recovered from immunosuppressed mice had significantly more eggs in the uterus than those from immunocompetent controls.
Ducks and chicks were not susceptible to experimental
G. seoi infection; the worm recovery rate at day 7 PI was 1.3% and 0.0%, respectively (
Lee et al., 1997). It was an unexpected result, since the gymnophallid flukes are generally known to be avian parasites (
Schell, 1985), and the Palearctic oystercatcher
Haematopus ostralegus caught from the endemic area of
G. seoi, was found to harbor adult flukes (
Ryang et al., 2000). It was thus suggested that the susceptibility of avian hosts to
G. seoi infection might be different among different species of birds.
In this connection, 7 species of wild birds were caught and examined for their susceptibility to experimental
G. seoi infection (
Ryang et al., 2001). They included the Kentish plover (
Charadrius alexandrinus), Mongolian plover (
C. mongolus), grey plover (
Plurialis squatarola), dunlin (
Calidris alpina), great knot (
C. tenuirostris), black-tailed gull (
Larus crassirostris), and mallard (
Anas platyrhynchos). It was impossible to catch the Palearctic oystercatcher alive. Each bird was inoculated orally with 300 or 1,000 metacercariae of
G. seoi, and killed at days 2, 4, and 6 PI to recover worms.
The recovery of
G. seoi varied considerably among different species of birds (
Ryang et al., 2001). Through the experimental period of 6 days, the 3 species of plovers exhibited significantly higher worm recovery than the other 4 species of birds. Among the plovers, the greatest number of worms was recovered from the Kentish plover (56.0% in worm recovery), followed by the Mongolian (49.3%) and grey plovers (32.3%). No worms were recovered at day 6 PI from the great knot, dunlin, mallard, and black-tailed gull. In the Kentish plover and Mongolian plover, 70-80% of worms was recovered from the anterior half of the small intestine. The results indicate that the plovers are highly susceptible to
G. seoi infection, and may be a definitive host in nature.
Among the 3 plover species, the growth and development of
G. seoi was best in the Kentish plover and fairly good in the other 2 species of plovers (
Ryang et al., 2001). The ovary, testes and vitellaria were larger in worms recovered from the Kentish plover than in those from the other two species of plovers. Eggs were present in the uterus of 2-day-old worms (8-10 eggs per worm), with little difference in numbers among the plover species. By day 4 PI, the number of eggs increased to 23-30 per worm, without significant differences among the plover species. At day 6 PI, however, the increase in the number of eggs was greater in worms recovered from the Kentish plover than in those from the other 2 species. The results indicated that, among the 3 plover species, the Kentish plover was the most susceptible host for
G. seoi.
It is interesting to note that ICR and BALB/c mice retained many
G. seoi worms at day 3 PI, but most were expelled before day 7 PI (
Lee et al., 1997). The worms at day 3 PI were adults containing uterine eggs (
Chai et al., 1999). This can be regarded as spontaneous expulsion of the parasite after temporary establishment in the host, and probably caused by the immune response of the host.
However, the mucosal immune mechanisms leading to expulsion of intestinal helminths have been known to be complex. Studies with mast-cell deficient W/W
v mice provided strong evidence for the involvement of mucosal mast cells in expulsion of
Strongyloides ratti (
Abe and Nawa, 1987;
Abe et al., 1992,
1993). By contrast, in
Nippostrongylus brasiliensis infection, goblet cells were found to exert an important role in the expulsion of worms (
Ishikawa et al., 1993). On the other hand, no goblet cells nor mast cells played important roles in the host defense against
Neodiplostomum seoulense infection (
Chai et al., 1998b).
Exact immune effector mechanisms implicated in the expulsion of
G. seoi from the mouse intestine have not been documented until present. However, it is strongly suggested that goblet cells in the mucosa would be one of the important effectors responsible for the short-time parasitism of
G. seoi in rodent hosts (
Chai et al., 2001b). This suggestion is supported by the strong goblet cell responses in immunocompetent mice during days 3-7 PI, and only a small number of flukes retained in the intestines at days 7-14 PI. It is further supported by the minimal degree of goblet cell hyperplasia in immunosuppressed mice, with much more flukes surviving in the intestine. The detailed mechanisms of goblet cell involvement in the expulsion of
G. seoi, however, remain to be determined.
PATHOGENICITY AND HISTOPATHOLOGY
Pathogenicity of G. seoi to man and animals
It has been documented that
G. seoi is not highly pathogenic to C3H/HeN mice (
Chai et al., 2001b), compared with other species of intestinal flukes such as
Metagonimus yokogawai and
Echinostoma hortense (
Chai and Lee, 1990). The mucosal damage caused by
G. seoi in those mice, which will be discussed later in detail, was generally not severe. However, in man, the pathogenicity of
G. seoi is considerably high; the first human case suffered from severe gastrointestinal symptoms, with signs of acute pancreatitis (
Lee et al., 1993). Infected people in endemic areas complained of variable degrees of abdominal pain and diarrhea (
Lee et al., 1994). In avian hosts, the pathogenicity has not been well documented.
Differences in the pathogenicity of
G. seoi to different hosts, if there are any, may be due to several reasons. These reasons would include differences in their genetic susceptibility to
G. seoi infection (
Lee et al., 1997;
Ryang et al., 2001), and thereby, different worm burdens in different hosts. Also included would be differences in the habitat of
G. seoi in hosts, and variations in immune responses and immunopathological mechanisms in different hosts. In immunocompetent C3H/HeN mice,
G. seoi worms did not invade beyond the mucosa of the small intestine, but they invaded the submucosa in immunosuppressed C3H/HeN mice (
Chai et al., 2001b). In the latter case, there is a possibility for
G. seoi eggs to be transferred to remote organs and provoke egg granuloma in the immunosuppressed hosts.
With regard to the mechanisms of eliciting pathogenicity by intestinal flukes, mechanical and chemical stimuli have been suggested as responsible factors from the parasite side (
Chai and Lee, 1990). In the case of
G. seoi, mechanical irritation by the flukes was considered to be an important mechanism (
Chai et al., 2001b). In intestinal sections, many adult flukes were sucking the root of villi, with the large oral suckers, and villi nearby the flukes were pressure-atrophied, and some areas showed complete loss of villi. Cysteine proteinases were isolated from metacercariae and adults (
Choi et al., 1998a,
1998b). However, it was speculated that they might be involved in nutrient uptake and immune evasion from the host, but not in eliciting pathogenicity to the host.
Intestinal fluke infection may affect host intestinal histopathology. Many studies have documented villous atrophy and crypt hyperplasia with inflammatory reactions in the villous stroma and crypts (
Chai and Lee, 1990). The flukes studied included
Metagonimus yokogawai (
Chai, 1977;
Lee et al., 1981;
Kang et al., 1983;
Chai et al., 1994a,
1995),
Neodiplostomum seoulense (
Lee et al., 1985),
Pygidiopsis summa (
Seo et al., 1986), and
Echinostoma hortense (
Lee et al., 1990).
Intestinal histopathology elicited by
G. seoi was reported using C3H/HeN mice (
Chai et al., 2001b). Whereas uninfected control mice revealed the normal contour of the mucosa of the small intestine, the intestines of
G. seoi-infected mice showed villous atrophy and crypt hyperplasia in the duodenum and jejunum, with inflammatory reactions in the villous stroma and crypt. The pathological changes, however, was generally not severe, and the intestinal integrity was restored after day 14-21 PI.
Goblet cell hyperplasia was remarkable along the villous epithelial layers, especially in the jejunum (
Fig. 22) (
Chai et al., 2001b). The adult flukes of G. seoi were characteristically seen pinching and sucking the epithelial layer of villi with their oral suckers (
Fig. 23).
One of the most important reasons for the mild histopathology seems to be the small number of flukes which actually parasitized in the small intestine of immunocompetent mice (
Chai et al., 2001b). For example, the worm recovery of G. seoi from immunocompetent C3H/HeN mice was reported to be about 12% at day 7 PI (
Lee et al., 1997). When 300 metacercariae were given to each mouse, only about 36 adult flukes survived in the small intestines. A small number of flukes therefore do not appear to cause severe histopathological changes. Another reason may be the relatively low pathogenicity of
G. seoi compared to other intestinal flukes like echinostomes, which caused severe pathology with ulceration of the mucosa and bleeding from the infected sites (
Lee et al., 1990;
Chai et al., 1994b).
A striking observation among the histopathological features in the small intestines of
G. seoi-infected mice was the strong proliferation of goblet cells on the villous epithelia (
Chai et al., 2001b). This feature is of considerable significance for understanding the host-parasite relationships in
G. seoi infection, since goblet cells have been reported an important effector for expulsion of intestinal helminths; e.g.,
Nippostrongylus brasiliensis (
Nawa et al., 1994;
Onah and Nawa, 2000) and
Echinostoma trivolvis (
Fujino et al., 1993).
The effects of immunosuppression on the intestinal histopathology elicited by
G. seoi were also studied (
Chai et al., 2001b). Uninfected C3H/HeN mice immunosuppressed with prednisolone revealed nearly normal contour of the small intestinal mucosa. By contrast, in C3H/HeN mice, immunosuppressed and infected with 300 metacercariae of
G. seoi, the histopathological features were similar to those observed in immunocompetent mice. The destruction of villi adjacent to the flukes was, however, generally more severe than in immunocompetent mice. Many juvenile or adult flukes were seen sectioned occupying the intervillous spaces. They were seen pinching the root of the villus with the large oral suckers. Goblet cell hyperplasia was generally not remarkable. Villi adjacent to the flukes were destroyed, and some areas showed complete loss of villi. It was suggested that immunosuppression enhanced survival of many flukes in the host intestinal mucosa, and facilitated mechanical irritation by the flukes on the neighboring villi and crypts. Owing to immunosuppression, however, host systemic immune responses must have been compromised, and mucosal inflammation and pathology other than destruction of villi appeared to be generally mild.
GROWTH AND DEVELOPMENT
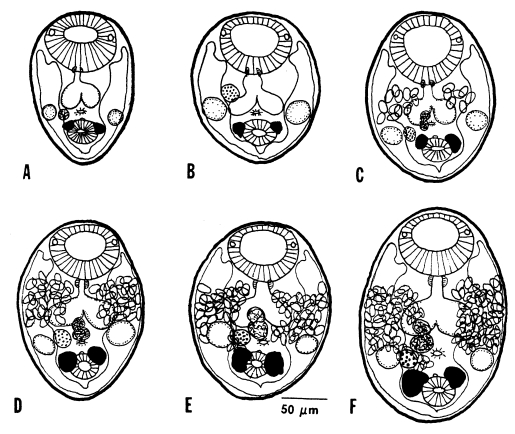
Growth and development of worms in mice
The growth and development of
Gymnophalloides seoi were studied in C3H/HeN mice and effects of immunosuppression of mice on the worm development were observed (
Chai et al., 1999). The worms attained sexual maturity by day 3 PI with eggs in the uterus, and worm dimensions and the number of uterine eggs increased until day 14 PI in immunosuppressed mice (
Figs. 25-
26). In immunocompetent mice, however, the worms attained their maximum growth on day 5 PI, then few worms were retained in the intestine, and they showed no more recognizable growth in size. Worms from immunosuppressed mice were significantly larger in size than those from immunocompetent mice.
Genital organs such as the ovary, testes, and vitellaria, which were already developed in the metacercarial stage, developed quickly to maturity within days 2-3 PI, as shown by the appearance of uterine eggs on day 3 PI (
Figs. 25-
26). The number of eggs increased gradually in accordance with the age of worms (
Fig. 26). Worms recovered from immunosuppressed mice had more eggs in the uterus than immunocompetent mice. The seminal vesicle and seminal receptacle were not yet recognizable in 1-day worms, but seen to have developed by day 3 PI (
Fig. 25).
Based on the successful growth and development of worms, C3H/HeN mice could be considered an optimum laboratory host for
G. seoi (
Chai et al., 1999). However, the size of worms even from immunosuppressed C3H/HeN mice as well as the number of uterine eggs were smaller than those recovered from humans (
Lee et al., 1993).
The duration for trematodes to be ovigerous has been reported variable. In the case of
Neodiplostomum seoulense, for example, primordia of reproductive organs were not recognizable in the metacercarial stage, and the ovary appeared 3 days after experimental infection in rats, and they became ovigerous on day 5 PI (Hong, 1982). On the other hand, in
Heterophyopsis continua, the ovary and testes were already present in the metacercarial stage, and the presence of uterine eggs required only 3 days (
Hong et al., 1990).
Gymnophalloides seoi attained sexual maturity quickly, in 3 days, in C3H/HeN mice (
Chai et al., 1999). This may be due to the early development of the ovary, testes, and vitellaria in the metacercarial stage (
Lee et al., 1995). Sperms and ova were produced shortly after infection in the definitive host, followed by egg production. It was, therefore, necessary to cultivate
G. seoi metacercariae into egg-producing adults in vitro.
In vitro cultivation of worms
Attempts have been made to cultivate parasitic helminths including various species of trematodes in the laboratory from metacercariae or juvenile flukes to sexually mature adults (
Silverman and Hansen, 1971;
Taylor and Baker, 1987;
Seo, 1989). Among the family Gymnophallidae, cultivation of
Parvatrema timondavidi was tried, and a considerable degree of worm maturation was attained (
Yasuraoka et al., 1974). In vitro cultivation of
G. seoi from metacercariae to egg-producing adults was tried using NCTC 109 medium, and the cultured worms were compared with those recovered from experimentally infected C3H mice (
Kook et al., 1997).
Under culture condition of 37℃/5% CO
2, there was no recognizable growth and development of worms, although over 95% of worms were alive until day 5 (
Kook et al., 1997). The average length of worms on day 2 to day 5 was not significantly different from that of metacercariae. In worms cultured under this condition sexual maturation was not recognizable without eggs in their uteri. By contrast, under the condition of 41℃/5% CO
2 or 41℃/8% CO
2, sexual maturation of worms, particularly in terms of the number of uterine eggs, was much better than those cultured at 37℃/5% CO
2. All of the reproductive organs were well developed, and a few eggs appeared in the uterus on day 2 after culture. The average number of eggs per worm was 2.3 on day 2, 15.8 on day 3, and 20.7 on day 5 after culture at 41℃/5% CO
2. The optimum temperature for cultivation of
P. timondavidi was 41℃ rather than at 37℃, according to Yasuraoka et al. (
1974).
The preferred temperature of 41℃ for both
P. timondavidi and
G. seoi is well correlated with their natural life cycles; birds taking the role for a natural final host (
Yasuraoka et al., 1974;
Ryang et al., 2000). However, it should not be an essential condition for growth and maturation of
G. seoi, considering that many humans are infected with this fluke in endemic areas (
Lee et al., 1994), and C3H mice are a fairly good laboratory host (
Lee et al., 1997;
Chai et al., 1999).
Metacercariae of
G. seoi were successfully cultured in vitro, developing into adults containing mature reproductive organs and eggs (
Kook et al., 1997). The extent of growth and development of worms, however, was not comparable with those recovered from C3H/HeN mice (
Chai et al., 1999) or from human infections (
Lee et al., 1993,
1994). It is suggested that in vitro conditions are far from satisfactory for the full development of worms, compared with man and animals.
Reproduction of
G. seoi in man has been estimated in terms of the eggs per gram of feces per worm (EPG/worm) and eggs per day per worm (EPD/worm) (
Chai et al., 2000). The EPG/worm obtained from 21 human infections ranged widely from 0.04 to 0.77 (av. 0.23) and the daily egg output of
G. seoi was estimated at about 2-84 per worm, much smaller than other parasites.
The wide range of the daily egg output of
G. seoi was due to density-dependent constraints on the parasite fecundity, as observed in nematodes such as
Ascaris lumbricoides (
Anderson and May, 1982) and trematodes such as
Metagonimus yokogawai (
Seo et al., 1985). Therefore, equations applicable to express the relations between the worm burden (X) and worm fecundity (Y) were introduced; Y=a·X
b or Y=a·e
-bx, where 'a' and 'b' are constants determining the degree of constraints (
Anderson and May, 1982). Using the latter equation, a regression curve was drawn as Y=0.42·e
-1.20 (r=0.49) for
G. seoi, and theoretical values of the EPG/worm and EPD/worm were calculated as shown in the above.
In general, the egg laying capacity of trematodes is much lower than that of nematodes. For example, the egg laying capacity of
A. lumbricoides in the human host is known to be 90,000-280,000 eggs per day per mature female (
Chai et al., 1981). On the other hand,
Clonorchis sinensis deposit smaller numbers of eggs, 360 eggs per day per worm in experimental rats (
Seo, 1958), and probably a little more in the human host. In the case of small intestinal trematodes, the egg laying capacity is much lower than that of
C. sinensis. For example, the daily number of eggs produced per
M. yokogawai adult was estimated at 14-64 eggs in the human host, with remarkable density-dependent con-straints on the worm fecundity (
Seo et al., 1985). The density-dependent constraints in
G. seoi were more remarkable than in
M. yokogawai.
PREVALENCE AND GEOGRAPHICAL DISTRIBUTION
Metacercarial infection in oysters
The prevalence and intensity of
Gymnophalloides seoi (metacercariae) infections were different in oysters collected from different localities (
Lee et al., 1995a,
1996;
Sohn et al., 1998;
Lee et al., 1999). The greatest infection rate and intensity was observed in oysters collected from areas of Shinan-gun. Among those areas, Aphaedo (island), where the first patient resided (
Lee et al., 1993), showed the highest infection rate and the greatest metacercarial density in oysters. The infection rate was 100% among oysters collected, and the average number of metacercariae per oyster was 610 (
Lee et al., 1995a). In another study on Aphaedo, the infection rate of oysters was also 100%, and the intensity of infection was 785.9 metacercariae per oyster (
Lee et al., 1996). A third study on the same island revealed a year-round average prevalence of 88.6% in oysters, and an average metacercarial density per oyster of 1,339 (
Sohn et al., 1998).
Oysters collected from other islands of Shinan-gun showed variable results in prevalence and intensity of infection (
Lee et al., 1996). Nine of 12 islands examined were found to have infected oysters. The infection rate was the highest in Anchwado (100%), followed by Amtaedo (67.5%), Chungdo (58.3%), Jido (54.5%), Palgumdo (27.0%), Jangsando (14.1%), Haeuido (8.1%), Shineuido (1.7%), and Dochodo (1.3%). The metacercarial density was the highest in oysters from Chungdo (203.0 metacercariae per oyster), followed by oysters from Anchwado (67.6), Amtaedo (62.9), and 6 other islands (less than 20.1). The infected oysters were found mainly in the coastal islands of Shinan-gun. Oysters from remote islands tended to have low-grade infections or none at all.
Besides Shinan-gun,
G. seoi-infected oysters have been found from western coastal areas (
Lee et al., 1996;
Sohn et al., 1998;
Lee et al., 1999). For example, one coastal area of Buan-gun revealed one infected oyster among 50 examined (2.0%), and the number of metacercariae in the oyster was 15 (
Lee et al., 1996). Infected oysters were also found on 2 islands of Gunsan-shi, Chollabuk-do; the prevalence was 100% on Munyodo island (53.5 metacercariae per oyster, n = 50), and 83.3% on Sunyudo island (12.6 per oyster, n = 30) (
Sohn et al., 1998). In another study on Munyodo island (
Lee et al., 1999), the infection rate was 80.8%, and the average metacercarial density per oyster was 197 (n = 52). In addition, Yubudo island (Sochon-gun, Chungchungnam-do) and Chumoondo island (Kangwha-gun, Inchon-shi) had infected oysters (
Sohn et al., 1998).
It has been suggested that seasonal variation is not evident with metacercarial stages of trematodes in general; it is correlated with longevity of metacercariae in the host (
Erasmus, 1972). Therefore, the incidence would rise in summer after exposure to a second cercarial population. Seasonal variation of metacercarial density was reported in a gymnophallid trematode; a slight increase in the number of
Lacunovermis macomae metacercariae was observed in summer season in the extrapallial space of
Macoma balthica (
Pekkarinen, 1983).
In the case of
G. seoi, seasonality of the prevalence and metacercarial density in oysters has not been studied except for the work of Sohn et al. (
1998). They observed lower infection rate of oysters during the winter season (December-February) than in other seasons. The lowest prevalence was found in January (25%), followed by February (40%) and December (85%). In other months 95-100% of oysters were infected with the metacercariae. With metacercarial density, however, no significant seasonal differences were noted; 1,095-1,130 per oyster during winter, and 761-2,077 during other seasons.
The home village of the first human case (
Lee et al., 1993), Aphaedo island, Shinan-gun, was surveyed to determine the prevalence and intensity of infection with
G. seoi (
Lee et al., 1994). A total of 98 inhabitants (male 44, female 54), 3 to 81 years of age, were studied. The egg positive rate of
G. seoi was 49.0% (48/98) (
Table 4); 50.0% in males and 48.1% in females. The age-prevalence was not remarkable. Individuals positive for
G. seoi eggs were treated with 10 mg/kg single dose of praziquantel followed by purgation with 20-30 g magnesium sulfate (MgSO
4) to recover the adult flukes. The measurement of individual worm burden was completed in 15 cooperative people. The number of
G. seoi specimens collected from each case was in the range 106-26,373, with an average value of 3,326 per infected case (
Table 4). Their study (
Lee et al., 1994) established clear evidence that
G. seoi is a human intestinal trematode occurring under natural conditions. These findings completely ruled out the possibility of accidental infection of the first human case (
Lee et al., 1993).
After the village on Aphaedo, Shinan-gun was proved to be a highly endemic area of
G. seoi infection in 1989 (
Lee et al., 1994), field studies have been conducted in 1997 and 2000 to know the persistence of the endemicity in this village (
Chai et al., 2000). In each survey (including the one in 1989), mass treatments were undertaken with 10 mg/kg single dose of praziquantel on the whole villagers. In 1997, the egg positive rate of
G. seoi was 71.3% (67/94), much higher than in 1989, and it returned back to 72.0% (77/107) in 2000.
In 1997, the measurement of individual worm burdens (minimum confirmed number) was completed in 31
G. seoi-infected cases, who cooperated in the anthelmintic treatment and purgation (
Chai et al., 2000). The number of
G. seoi specimens collected from each case was in the range 94-69,125, with average 10,344 worms per infected case (
Table 4). In 1997, the total sum of the
G. seoi EPG was 63,928 among 63 egg positive cases, with average 1,015 EPG per case. In 2000, the total sum of
G. seoi EPG was 22,962 among 65 egg positive cases, with an average value of 353 per case, significantly lower than that in 1997 (
Table 4). The number of cases with lower EPGs than 1,000 was higher in 2000 than that in 1997.
As shown, the egg positive rate of
G. seoi and individual worm burdens were both surprisingly high in two repeated examinations (1997 and 2000), and represented persistent endemicity of this fluke in the surveyed village (
Chai et al., 2000). With respect to the egg positive rate, the rates in 1997 and 2000 were rather higher compared to the previously reported rate of 49.0% in the same village (
Table 4). The individual worm burdens in 1997 were significantly higher than in 1989 (
Lee et al., 1994).
Such heavy infections with
G. seoi must have been undoubtedly caused by frequent and repeated consumption of raw oysters. The density of metacercariae in oysters collected near the endemic village averaged 785.9 (
Lee et al., 1995a). Therefore, several oysters eaten raw could lead to infection with more than 1,000 worms. The high intensity of metacercarial infection in oysters, together with eating habit of raw oysters, could be responsible for the persistence of
G. seoi infection in this coastal village.
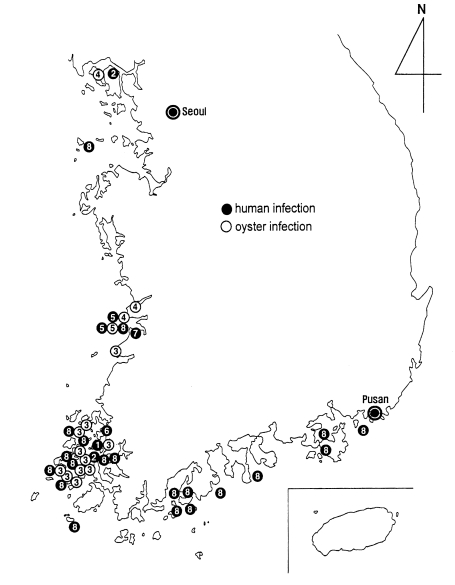
A nationwide survey was performed on the prevalence and intensity of
G. seoi infection among inhabitants residing on a total of 45 islands on the Yellow (western) Sea and South (southern) Sea, the Republic of Korea (
Chai et al., 2001a). Of 4,178 fecal specimens examined, 160 (3.8%) appeared positive for eggs of
G. seoi. The egg positive cases of
G. seoi were found from 22 (48.9%) of 45 islands surveyed. The positive rate was the highest in Amtaedo (25.3%), followed by Cheungdo (25.0%), Anchwado (20.9%), Munyodo (13.3%), Shinshido (12.9%), Sonyudo (10.3%), Pyongildo (9.3%), Kogumdo (8.8%), Kohado (5.9%), Dallido (5.8%), and Kogumdo (5.4%). Islands which revealed lower prevalences than 5% were Keogumdo (2.2%), Tochodo (2.1%), Hauido (2.1%), Hajodo (1.9%), Naenarodo (1.9%), Soyado (1.8%), Sandaldo (1.5%), Tolsando (1.1%), Pikeumdo (1.0%), Kajodo (1.0%), and Kadokdo (0.8%).
Among the egg positive cases of
G. seoi, the EPG per individual ranged from 24 to 4,392, and averaged 154.4 (n = 153) (
Chai et al., 2001a). According to different islands, the average EPG was the highest in Shinshido (696), followed by Kogumdo (288), Sonyudo (254), Pyeongildo (240), Sorokdo (216), Soyado (144), Naenarodo (120), Amtaedo (111), Tochodo (72), Keogumdo (72), Dalido (56), Anchwado (54), Cheungdo (48), Munyodo (45), Kohado (30), and Hauido (24). The egg positive rate varied also by the age groups. The highest rate was observed in 60-69 year olds (5.3%), followed by 50-59 years (5.1%), 70-79 years (4.9%), over 80 years (4.2%), 40-49 years (3.4%), 20-29 years (3.1%), 30-39 years (0.8%), and 0-19 years (0.0%). Females (4.1%) showed higher positive rate than males (3.5%).
Among the 22 islands, 2 (Munyodo and Sunyudo) were known endemic areas (
Lee et al., 1999), and at least 11 were new endemic areas of human infections with
G. seoi (
Chai et al., 2001a). Areas with over 20.0%, 10.0-19.9%, and 5.0-9.9% in the prevalence were designated as high, moderate, and low endemic areas, respectively. Three islands (Amtaedo, Cheungdo, and Anchwado) were highly endemic, 3 (Shinshido, Munyodo, and Sunyudo) were moderately endemic, and 5 (Kohado, Dallido, Pyeongildo, Keogumdo, and Kogumdo) were low endemic areas.
Among the 20 newly found areas of human
G. seoi infection, included were 11 islands not far from the previously known areas (
Chai et al., 2001a). Amtaedo, Cheungdo, Anchwado, Hauido, Dochodo, Pikeumdo (Shinan-gun), Kohado, Dallido (Mokpo-shi), and Hachodo (Chindo-gun), are all close to Aphaedo (Shinan-gun), the most well known endemic area (
Lee et al., 1994). Shinshido is a small island close to the known villages in Puan-gun (
Chai et al., 1998) and also near Munyodo and Sunyudo, the known areas (
Lee et al., 1999). Soyado is a little southwest to Kangwhado island, where a human case was reported (
Lee et al., 1995b). The remaining 9 islands were located on the southern sea (Chollanam-do, Kyongsangnam-do, or Pusan-shi), very remote from the known endemic areas (
Chai et al., 2001a). A group of 4 islands (Pyeongildo, Keogumdo, Kogumdo, and Sorokdo), very close to one another, are of considerable interest; the prevalence at present being low, attention should be paid on these islands in the future. Still remained 5 islands are scattered, and only a few infected cases were found on each island.
The presence of human infections on the southern islands is interesting, since oystercatchers are few along the southern coasts (
Won, 1993), and oysters caught from southern coasts were free from
G. seoi infection (
Lee et al., 1996). Several possibilities could be raised. First, the eggs detected from humans on southern islands might not be
G. seoi but eggs of other gymnophallid species. It is worthwhile to note that more than 2 gymnophallid species,
Parvatrema timondavidi (
Yu et al., 1993) and
Meiogymnophallus sp. (
Lee et al., 1996), exist in the Republic of Korea, although their capability of infecting humans is yet unknown. The former is transmitted by a marine bivalve (
Yu et al., 1993), and the latter by the razor clam (
Lee et al., 1996), both of which are eaten raw by the local people.
Second, if they really are
G. seoi eggs, there may exist a new second intermediate host other than the oysters. In
G. heardi (
Ching, 1995b), a species related to
G. seoi, a pulmonate gastropod
Melampus bidentatus plays the role for a second intermediate host. A third possibility is that there may be new definitive hosts other than the oystercatchers and man. It may be possible, since the host specificity of
G. seoi for the definitive host is considerably low. Possible new hosts include mice, rats, hamsters, gerbils, and cats (
Lee et al., 1997), and birds such as plovers (
Ryang et al., 2001). In
G. heardi, a rodent
Oryzomys palustris is the natural definitive host (
Ching, 1995b).
Based on the surveys on oyster (
Lee et al., 1995a,
1996;
Sohn et al., 1998;
Lee et al., 1999) and human infections (
Lee et al., 1994;
Chai et al., 1997,
1998,
2001a), the geographical distribution of
G. seoi is presented in a figure (
Fig. 27). The distribution is unexpectedly wide, from northwestern to southeastern coastal islands of the Republic of Korea. However, it should be emphasized that the infection is most highly prevalent on the coastal islands of Shinan-gun. Low-grade endemicity occurs in adjacent localities such as Muan-gun and Buan-gun, and islands (Munyodo and Sunyudo) of Gunsan-shi, Wando-gun, and Kohung-gun.
It is difficult to explain properly the specificity in the geographical location of this fluke infection. Migrating birds such as oystercatchers are suspected as the major natural definitive hosts (
Ryang et al., 2000). If so, the distribution of highly endemic areas should have been wider than observed. There is a possibility that the major definitive host for
G. seoi might be humans, not migratory birds, or the snail intermediate host shedding the cercariae might be distributed only around the endemic areas, which could explain the limited geographical distribution.
Nevertheless, it is taken for granted that G. seoi be distributed in areas where oysters and oystercatchers are available, and human infections may be present, where oysters are consumed raw by the local people. It should be ruled out that this parasite is distributed in other coastal areas of Korea, and even in neighboring countries such as China, Japan, and eastern coasts of Russia.
CLINICAL ASPECTS OF HUMAN INFECTIONS
Clinical manifestations
In intestinal fluke infections, clinical complaints are mostly gastrointestinal, and include abdominal pain, diarrhea, and indigestion (
Chai and Lee, 1990). General symptoms such as fever, anorexia, weight loss, easy fatigue, and weakness may also be found. The symptoms, however, if not heavily infected, are generally not severe and nonspecific in characters.
In the case of
G. seoi infection, the clinical manifestations of patients are variable. For example, the first patient infected with
G. seoi experienced repeated episodes of epigastric discomforts, indigestion, and diarrhea (
Lee et al., 1993). Laboratory studies revealed elevated serum and urine amylase levels, increased serum alkaline phosphatase activity, and a slight to moderate degree of eosinophilia (3-12%). The tentative clinical diagnosis was acute pancreatitis or acute cholecystitis. However, 5 days after the treatment of
G. seoi infection, the epigastric pain and diarrhea completely disappeared, and serum and urine amylase levels returned to their normal levels. It was suggested that the patient suffered from an intestinal, gallbladder or pancreatic duct infection with
G. seoi.
On the other hand, two interesting cases of
G. seoi infection were reported (
Lee et al., 1995b). They were accompanied by diabetes mellitus, and thereby certain relations with
G. seoi infection were suspected. Some patients infected with
G. seoi in a highly endemic area in Aphaedo (
Lee et al., 1994) complained of symptoms such as thirst, polydipsia, and polyuria (
Chai et al., 2000), that may occur in patients with diabetes mellitus. Their blood and urine glucose levels were, however, within normal limits.
Invasion of
G. seoi into the pancreatic duct or other organs of humans should be considered. However, there has been no experimental evidence on extraintestinal infection by
G. seoi. The infections have been generally mild (
Lee et al., 1994;
Chai et al., 2000). In a questionnaire study, a half of the
G. seoi-infected persons experienced mild degrees of numbness, amblyopia, weight loss, epigastric and abdominal pain, indigestion, constipation, diarrhea, and weakness (
Chai et al., 2000). Prompt treatment was not required. The symptoms were not necessarily correlated with the level of individual worm burdens.
The diagnosis of human
G. seoi infection is not easy. First, the eggs are too small, only 0.019-0.025 mm in length, smaller than those of Clonorchis sinensis or
Metagonimus yokogawai, and have a very thin and transparent shell (
Lee et al., 1993,
1994). The eggs of
G. seoi are not readily detected in routine fecal examinations performed by formalin-ether sedimentation or cellophane thick smear techniques, and may be easily overlooked, if examined by an inexperienced technician. They could be misdiagnosed as an air bubble or other artifacts especially in thick smears.
Secondly, the egg laying capacity of
G. seoi is very low, compared with other intestinal parasites such as
A. lumbricoides (
Chai et al., 1981). Unless heavily infected, detection of eggs in fecal examinations is difficult, because of low egg appearance in the feces. The daily output of
G. seoi was estimated as only 2-84 eggs per worm in the human host (
Chai et al., 2000). Unless more than 100 worms are present, less than 8,400 eggs would be present in the stool. In this case, the EPG would be only 42 (daily stool weight; 200 g). This value means appearance of only 1-2 eggs on the whole field of a fecal smear made by the Kato-Katz technique (41.7 mg of feces/smear).
Even if G. seoi (gymnophallid) eggs were detected in the feces, the specific diagnosis should be made after identification of adult flukes. Different species of gymnophallid flukes may represent similar egg morphology. The method for recovery of adult flukes from humans is as follow. Briefly, the patient positive for gymnophallid eggs is given orally with praziquantel 10 mg/kg in single dose, an hour later followed by purgation with 20-30 g magnesium salts with a large quantity of water. Within 2 to 3 hours, the patient would undergo diarrhea. The diarrheic stool should be thoroughly collected, washed several times in water and sedimented. The sediment should be examined for flukes under a stereomicroscope.
Treatment
The treatment of human
G. seoi infection can be successfully performed by giving single oral dose of 10 mg/kg praziquantel (
Lee et al., 1993,
1994;
Chai et al., 2000). Albendazole may also be effective against
G. seoi infection.
PREVENTION AND CONTROL
Prevention
Prevention from G. seoi infection is the avoidance of consuming infected oysters, raw or improperly cooked. Particularly consumption of oysters collected around the endemic areas should be avoided.
Control measures
Two studies have been reported as control measures; one is irradiation of oysters to control infectivity of the metacercariae (
Chai et al., 1996). The other is repeated chemotherapy of the people in an endemic area with praziquantel (
Chai et al., 2000).
To control infectivity of the metacercariae by irradiation, two experimental groups were prepared; metacercaria (isolated)-irradiation group and oyster-irradiation group (
Chai et al., 1996). The irradiated metacercariae and those isolated from irradiated oysters were given to C3H mice, and worm recovery was performed at day 7 PI. In the former group, the worm recovery was significantly lower at irradiation doses higher than 200 Gy, and the number of intrauterine eggs significantly reduced at doses over 50 Gy. In the latter group, only 50 Gy significantly reduced both the worm recovery and number of uterine eggs. In the two groups, no worms were recovered at 1,000 Gy irradiation. It is concluded that irradiation of oysters with 200-1,000 Gy is effective to control infectivity of metacercariae, and could be adopted as a control measure for
G. seoi infection.
To evaluate the efficacy of repeated chemotherapy of people for the control of
G. seoi infection, the known endemic village on Aphaedo, Shinan-gun (
Lee et al., 1994) was selected, and the entire population was repeatedly treated with praziquantel (
Chai et al., 2000). However, as the chemotherapy was repeated every 3 years, no control efficacy was obtained;
G. seoi egg positive rate was 71.3% (67/94) in 1997, and returned to 72.0% (77/107) in 2000 (
Table 4). In contrast, the average number of eggs per gram of feces (EPG) was reduced from 1,015 in 1997 to 353 in 2000.
The mass chemotherapy interval was greatly shortened to be only 3 months after March 2000, and the prevalence decreased remarkably from 72.0% in March 2000, to 22.2% in June 2000, 24.8% in September 2000, and 12.5% in December 2000 (unpublished observation). The prevalence is expected to decrease to 10% or lower in March-April 2000.
CONCLUSION
Studies on Gymnophalloides seoi (Digenea: Gymnophallidae) and human infections were briefly reviewed. Southwestern coastal islands, including Shinan-gun, are the major endemic areas. The source of human infection is the oyster Crassostrea gigas. Man and the palearctic oystercatcher Haematopus ostralegus are natural definitive hosts. Laboratory animals such as gerbils, hamsters, and mice (especially C3H/HeN) are susceptible hosts. In mice, G. seoi inhabit the small intestine, pinching, and sucking the root of villi with the oral suckers. Human G. seoi infections have been found in at least 25 localities; 23 islands on the Yellow Sea and the South Sea, and 2 western coastal villages. The highest prevalence was found in a village of Aphaedo, Shinan-gun (49% of the villagers infected). The major symptoms were gastrointestinal. The infection can be diagnosed by recovery of eggs in the feces. Praziquantel is effective for treatment of human infections, but eating raw oysters should be avoided in endemic areas.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Sung-Tae Hong, Dr. Min-Ho Choi, Dr. Eun-Hee Shin, Mr. Eun-Taek Han, Dr. Jae-Hwan Park, and Mr. Jae-Lip Kim, Department of Parasitology, Seoul National University College of Medicine, who greatly helped our series of studies on G. seoi. Special thanks should be given to Dr. Yong-Suk Ryang, Department of Medical Laboratory Sciences, College of Health Sciences, Yonsei University, for his discovery of oystercatchers taking the role of a natural definitive host for G. seoi, and for conducting experimental infection of avian hosts. We also thank our colleages, Dr. Sung-Jong Hong, Department of Parasitology, College of Medicine, Chung-Ang University, Dr. Woon-Mok Sohn, Department of Parasitology, College of Medicine, Gyeong-Sang National University, Dr. Jae-Ran Yu, Department of Parasitology, College of Medicine, Kon-Kuk University, Dr. Jina Kook, Department of Parasitology, Yanbian Medical College (Jinlin, P.R. China), Dr. Min Seo, Department of Parasitology, College of Medicine, Dankook University, Dr. Yoon-Kyu Park, Department of Parasitology, College of Medicine, Inha University, and Dr. Sang-Mee Guk, Department of Parasitology, College of Medicine, Yonsei University, for their invaluable contributions for studies on G. seoi.
References
- 1. Abe T, Nawa Y. Reconstitution of mucosal mast cells in W/Wv mice by adoptive transfer of bone-marrow derived cultured mast cells and its effects on the protective capacity to Strongyloides ratti infection. Parasite Immunology 1987;9:31-38.
- 2. Abe T, Sugaya H, Yoshimura K. Different susceptibility to the IL-3 induced protective effects between Strongyloides ratti and Nippostrongylus brasiliensis in C57BL/6 mice. Parasite Immunology 1993;15:643-645.
- 3. Abe T, Sugaya H, Yoshimura K, Nawa Y. Induction of the expulsion of Strongyloides ratti and retention of Nippostrongylus brasiliensis in athymic nude mice by repetitive administration of recombinant interleukin-3. Immunology 1992;76:10-14.
- 4. Anderson RM, May RM. Population dynamics of human helminth infections: control by chemotherapy. Nature 1982;297:557-563.
- 5. Bennett CE. Electron microscope studies of Fasciola hepatica. XIII. Fine structure of newly excysted juvenile. Exp Parasitol 1975;34:85-99.
- 6. Bennett CE, Threadgold LT. Fasciola hepatica: Development of tegument during migration in mouse. Exp Parasitol 1975;38:38-55.
- 7. Chai JY. Study on Metagonimus yokogawai (Katsurada, 1912) in Korea. V. Intestinal pathology in experimentally infected albino rats. Seoul J Med 1977;20:104-117.
- 8. Chai JY, Chung WJ, Kook J, et al. Growth and development of Gymnophalloides seoi in immunocompetent and immunosuppressed C3H/HeN mice. Korean J Parasitol 1999;37:21-26.
- 9. Chai JY, Han MS, Seo M, Lee SH. Effects of gamma-irradiation on the survival and development of Gymnophalloides seoi in C3H mice. Korean J Parasitol 1996;34:21-25.
- 10. Chai JY, Hong ST, Lee SH, Lee GC, Min YI. A case of echinostomiasis with ulcerative lesions in the duodenum. Korean J Parasitol 1994b;32:201-204.
- 11. Chai JY, Hong ST, Lee SH, Seo BS. Fluctuation of the egg production amounts according to worm burden and length of Ascaris lumbricoides. Korean J Parasitol 1981;19:38-44. (in Korean).
- 12. Chai JY, Kim TK, Cho WH, et al. Intestinal mastocytosis and goblet cell hyperplasia in BALB/c and C3H mice infected with Neodiplostomum seoulense. Korean J Parasitol 1998b;36:109-119.
- 13. Chai JY, Kim J, Lee SH. Invasion of Metagonimus yokogawai into the submucosal layer of the small intestine of immunosuppressed mice. Korean J Parasitol 1995;33:313-321.
- 14. Chai JY, Kim IM, Seo M, et al. A new endemic focus of Heterophyes nocens, Pygidiopsis summa, and other intestinal flukes in a coastal area of Muan-gun, Chollanam-do. Korean J Parasitol 1997;35:233-238.
- 15. Chai JY, Lee HS, Hong SJ, et al. Intestinal histopathology and in situ postures of Gymnophalloides seoi in experimentally infected mice. Korean J Parasitol 2001b;39:31-41.
- 16. Chai JY, Lee GC, Park YK, et al. Persistent endemicity of Gymnophalloides seoi infection in a southwestern coastal village of Korea with special reference to its egg laying capacity in the human host. Korean J Parasitol 2000b;38:51-57.
- 17. Chai JY, Lee SH. Intestinal trematodes of humans in Korea: Metagonimus, heterophyids and echinostomes. Korean J Parasitol 1990;28(suppl.):103-122.
- 18. Chai JY, Park JH, Han ET, et al. A nationwide survey of the prevalence of human Gymnophalloides seoi infection on western and southern coastal islands in the Republic of Korea. Korean J Parasitol 2001a;39:23-30.
- 19. Chai JY, Seo BS, Lee SH. Study on Metagonimus yokogawai in Korea VII. Susceptibility of various strains of mice to Metagonimus infection and effect of prednisolone. Korean J Parasitol 1984;22:153-160.
- 20. Chai JY, Song TE, Han ET, et al. Two endemic foci of heterophyids and other intestinal fluke infection in southern and western coastal areas in Korea. Korean J Parasitol 1998a;36:155-161.
- 21. Chai JY, Yun TY, Kim J, Huh S, Choi MH, Lee SH. Chronological observation on intestinal histopathology and intraepithelial lymphocytes on the intestine of rats infected with Metagonimus yokogawai. Korean J Parasitol 1994a;32:215-221. (in Korean).
- 22. Ching HL. Life cycles of Lacunovermis conspicuus n. gen., n. sp. and Meiogymnophallus multigemmulus n. gen, n. sp. (Gymnophallidae: Trematoda) from Macoma inconspicua and diving Ducks from Vancouver, Canada. Proceed Helminthol Soc Wash 1965;32:53-63.
- 23. Ching HL. A redescription of Gymnophalloides tokiensis Fujita, 1925 (Trematoda: Gymnophallidae). Canadian J Zool 1972;50:1299-1302.
- 24. Ching HL. Evaluation of characters of the digenean family Gymnophallidae Morozov, 1955. Can J Fish Aquat Sci 1995a;52(Suppl. 1):78-83.
- 25. Ching HL. Four new species of gymnophallid digeneans from rice rats, willets, and molluscs in Florida. J Parasitol 1995b;81:924-928.
- 26. Choi MH, Chai JY, Lee SH. Purification and characterization of a 16-kDa cysteine proteinase of Gymnophalloides seoi (Gymnophallidae) metacercariae. J Parasitol 1998a;84:350-355.
- 27. Choi MH, Park WJ, Chai JY, Lee SH. Surface ultrastructure of metacercaria and adult of Gymnophalloides seoi (Digenea: Gymnophallidae). Korean J Parasitol 1995;33:289-296.
- 28. Choi MH, Park WJ, Park YK, Chai JY, Lee SH. Isolation and characterization of a 40 kDa cysteine protease from Gymnophalloides seoi adult worms. Korean J Parasitol 1998b;36:133-141.
- 29. David R, David C. Waders of the world. 1995, London, U.K.. Hamlyn Ltd..
- 30. Erasmus DA. The Biology of Trematodes. 1972, London, U.K.. Edward Arnold.
- 31. Erasmus DA, Ohman C. The structure and function of the adhesive organ in strigeoid trematodes. Ann NY Acad Sci 1963;113:7-35.
- 32. Erasmus DA, Ohman C. Electron microscope studies of the gland cells and host-parasite interface of the adhesive organ of Cyathocotyle bushiensis Khan, 1962. J Parasitol 1965;561:761-769.
- 33. Fujino T, Fried B, Tada I. The expulsion of Echinostoma trivolvis: worm kinetics and intestinal cytopathology in conventional and congenitally athymic BALB/c mice. Parasitology 1993;106:297-304.
- 34. Fujino T, Ishii Y, Choi DW. Surface ultrastructure of the tegument of Clonorchis sinensis newly excysted juveniles and adult worms. J Parasitol 1979;65:579-590.
- 35. Fujita T. Etudes sur les parasites de Phuitre comestible du Japon, Ostrea gigas Thunberg. Traduction accompagnee de notes, de diagnosis et d'une bibliographie, par Robert-Ph. Dollfus. Ann Parasit Hum Comp 1925;3:37-59.
- 36. Hong SJ, Chai JY, Lee SH. Surface ultrastructure of the developmental stages of Heterophyopsis continua (Trematoda: Heterophyidae). J Parasitol 1991;77:613-620.
- 37. Howard R, Moore A. A complete checklist of the birds of the world. 1991, London, U.K.. Academic Press.
- 38. Ip HS, Desser SS. Transmission electron microscopy of the tegumentary sense organs of Cotylogaster occidentalis (Trematoda: Aspidogastrea). J Parasitol 1984;70:563-575.
- 39. Ishikawa N, Horii Y, Nawa Y. Immune-mediated alteration of the terminal sugars of goblet cell mucins in the small intestine of Nippostrongylus brasiliensis-infected rats. Immunology 1993;78:303-307.
- 40. Ito A. Hymenolepis nana: Maturation in an immunosuppressed unnatural rat host. Exp Parasitol 1983;56:318-326.
- 41. Ito A, Kamiyama T. Cortisone-sensitive, innate resistance of Hymenolepis nana infection in congenitally athymic nude rats. J Helminthol 1987;61:124-128.
- 42. James BL. A new cercaria of the subfamily Gymnophallinae (Trematoda: Digenea) developing a unique "parthenita" in Littorina saxatilis (Olivi). Nature 1960;185:181-182.
- 43. James BL. The life cycle of Parvatrema homoeotecnum sp. nov. (Trematoda: Digenea) and a review of the family Gymnophallidae Morozov, 1955. Parasitology 1964;54:1-41.
- 44. Kang SY, Cho SY, Chai JY, Lee JB, Jang DH. A study on intestinal lesions of experimentally reinfected dogs with Metagonimus yokogawai. Korean J Parasitol 1983;21:58-73.
- 45. Kook J, Lee SH, Chai JY. In vitro cultivation of Gymnophalloides seoi metacercariae (Digenea: Gymnophallidae). Korean J Parasitol 1997;35:25-29.
- 46. Lee JB, Chi JG, Lee SK, Cho SY. Study on the pathology of metagonimiasis in experimentally infected cat intestine. Korean J Parasitol 1981;19:109-129.
- 47. Lee KJ, Park GM, Ahn YK, et al. Surveys on Gymnophalloides seoi infection in the Gogunsan Gundo (Islands) of Korea. Korean J Malacol 1999;15:121-125.
- 48. Lee SH, Chai JY, Hong ST. Gymnophalloides seoi n. sp. (Digenea: Gymnophallidae), the first report of human infection by a gymnophallid. J Parasitol 1993;79:677-680.
- 49. Lee SH, Chai JY, Lee HJ, et al. High prevalence of Gymnophalloides seoi infection in a village on a southwestern island of the Republic of Korea. Am J Trop Med Hyg 1994;51:281-285.
- 50. Lee SH, Choi MH, Seo M, Chai JY. Oysters, Crassostrea gigas, as the second intermediate host of Gymnophalloides seoi (Gymnophallidae). Korean J Parasitol 1995a;33:1-7.
- 51. Lee SH, Chai JY, Seo M, Choi MH, Kim DC, Lee SK. Two cases of Gymnophalloides seoi infection accompanied by diabetes mellitus. Korean J Parasitol 1995b;33:61-64.
- 52. Lee SH, Noh TY, Sohn WM, Kho WK, Hong ST, Chai JY. Chronological observation of intestinal lesions of rats experimentally infected with Echinostoma hortense. Korean J Parasitol 1990;28:45-52. (in Korean).
- 53. Lee SH, Park SK, Seo M, Guk SM, Choi MH, Chai JY. Susceptibility of various species of animals and strains of mice to Gymnophalloides seoi infection and the effects of immunosuppression in C3H/HeN mice. J Parasitol 1997;83:883-886.
- 54. Lee SH, Seo BS, Chai JY, Hong SJ. Study on Metagonimus yokogawai (Katsurada, 1912) in Korea VII. Electron microscopic observation on the tegumental structure. Korean J Parasitol 1984;22:1-10.
- 55. Lee SH, Sohn WM, Hong ST. Scanning electron microscopical findings of Echinochasmus japonicus tegument. Korean J Parasitol 1987;25:51-58.
- 56. Lee SH, Sohn WM, Hong SJ, et al. A nationwide survey of naturally produced oysters for infection with Gymnophalloides seoi metacercariae. Korean J Parasitol 1996;34:107-112.
- 57. Lee SH, Yoo BH, Hong ST, Chai JY, Seo BS, Chi JG. A histopathological study on the intestine of mice and rats experimentally infected by Fibricola seoulensis. Korean J Parasitol 1985;23:58-72.
- 58. Lumsden RD. Surface ultrastructure and cytochemistry of parasitic helminths. Exp Parasitol 1975;37:267-339.
- 59. Nawa Y, Ishikawa N, Tsuchiya K, et al. Selective effector mechanisms for the expulsion of intestinal helminths. Parasite Immunol 1994;16:333-338.
- 60. Onah DN, Nawa Y. Mucosal immunity against parasitic gastrointestinal nematodes. Korean J Parasitol 2000;38:209-236.
- 61. Pekkarinen M. Seasonal changes in condition and biochemical constituents in the soft part of Macoma balthica (Lamellibranchiata) in the Tvarminne brackish water area (Baltic Sea). Ann Zool Fennici 1983;20:311-322.
- 62. Pekkarinen M. Anatomy, histology and maturing of the metacercaria of Lacunovermis macomae (Trematoda: Gymnophallidae) from brackish-water Macoma balthica (southwestern Finland, Baltic Sea). Ann Zool Fennici 1984;21:481-498.
- 63. Pekkarinen M. Notes on a gymnophallid trematode, assumed to be Parvatrema affinis (Jameson & Nicoll, 1913) from Macoma balthica (L.) (Bivalvia). Ann Zool Fennici 1987;24:29-37.
- 64. Pekkarinen M, Ching HL. Comparisons of gymnophallid digeneans from North Pacific and Baltic clams, Macoma balthica (Bivalvia). J Parasitol 1994;80:630-636.
- 65. Rohde K, Watson NA. Ultrastructure of the tegument, ventral sucker and rugae of Rugogaster hydrolagi (Trematoda, Aspidogastrea). Intern J Parasitol 1992;22:967-974.
- 66. Ryang YS, Yoo JC, Lee SH, Chai JY. The Palearctic oystercatcher Haematopus ostralegus, a natural definitive host for Gymnophalloides seoi. J Parasitol 2000;86:418-419.
- 67. Ryang YS, Yoo JC, Lee SH, Chai JY. Susceptibility of avian hosts to experimental Gymnophalloides seoi infection. J Parasitol 2001;87:454-456.
- 68. Schell SC. Handbook of trematodes of North America, North of Mexico. 1985, Moscow, Idaho, USA. University Press of Idaho; pp 124-125.
- 69. Seo BS. Studies on the egg laying capacity and development of Clonorchis sinensis in albino rats. Seoul Nat Univ J (Nat Sci Ser C) 1958;7:1-15. in Korean.
- 70. Seo BS. Comparative growth and development of the metacercariae of Fibricola seoulensis (Trematoda: Diplostomidae) in vitro, in vivo and on the chick chorioallantois. Korean J Parasitol 1989;27:231-248.
- 71. Seo BS, Lee HS, Chai JY, Lee SH. Intensity of Metagonimus yokogawai infection among inhabitants in Tamjin river basin with reference to its egg laying capacity in human host. Seoul J Med 1985;26:207-212.
- 72. Seo BS, Cheong SK, Chai JY, Lee SH, Lee JB. Histopathology of small intestines of rats and mice experimentally infected with Pygidiopsis summa. Korean J Parasitol 1986;27:125-134.
- 73. Seo BS, Lee SH, Chai JY, Hong ST, Hong SJ. Studies on intestinal trematodes in Korea X. Scanning electron microscopic observations on the tegument of Fibricola seoulensis. Korean J Parasitol 1984;22:21-29.
- 74. Seo M, Chai JY, Lee SH. TEM ultrastructure of the tegumental layer of Gymnophalloides seoi (Digenea: Gymnophallidae). Korean J Parasitol 1995;33:165-172.
- 75. Sibly CG, Monroe BL. Distribution and taxonomy of birds of the world. 1990, New Haven. Yale University Press.
- 76. Silverman PH, Hansen EL. In vitro cultivation procedures for parasitic helminths: Recent advances. Adv Parasitol 1971;9:227-258.
- 77. Sohn WM, Ryang YS, Chai JY, Lee SH. Discovery of Gymnophalloides seoi metacercariae in oysters from islands of the West Sea known as the habitats of palearctic oystercatchers. Korean J Parasitol 1998;36:163-169.
- 78. Stunkard HW. New intermediate host for Parvatrema borealis Stunkard and Uzmann, 1958 (Trematoda). J Parasitol 1962;48:157.
- 79. Stunkard HW, Uzmann JR. Studies on the digenetic trematodes of the genera Gymnophallus and Parvatrema. Biol Bull (Woods Hole) 1958;115:276-302.
- 80. Szidat L. Uber eine Ungewohnlicke form parthenogenetischer vermehrung bei metacercarien einer Gymnophallus-Art aus Mytilus platensis, Gymnophallus australis n. sp. des Sudatlantik. Z Parazitenkd 1962;22:196-213.
- 81. Taylor AER, Baker JR. In vitro Methods for Parasite Cultivation. 1987, London & Orlando, Florida. Academic Press.
- 82. Won PO. A field guide to the birds of Korea. 1993, Seoul, Korea. Kyohack Co..
- 83. Yamaguti S. Studies on the helminth fauna of Japan. Part 25. Trematodes of birds IV. Jpn J Zool 1939;8:129-210.
- 84. Yamaguti S. Synopsis of digenetic trematodes of vertebrates. 1971, Tokyo, Japan. Keigaku Publishing Co..
- 85. Yasuraoka K, Kaiho M, Hata H, Endo T. Growth in vitro of Parvatrema timondavidi Bartoli, 1963 (Trematoda: Gymnophallidae) from the metacercarial stage to egg production. Parasitology 1974;68:293-302.
- 86. Yu JR, Chai JY, Lee SH. Parvatrema timondavidi (Digenea: Gymnophallidae) transmitted by a clam, Tapes philippinarum, in Korea. Korean J Parasitol 1993;31:7-12.
- 87. Yu JR, Park JY, Chai JY. Surface ultrastructure of Parvatrema timondavidi (digenea: Gymnophallidae) according to its developmental stages. Korean J Parasitol 1994;32:65-74.
Fig. 1
Gymnophalloides seoi (holotype), ventral view, adult fluke recovered from the first human infection. Reproduced from Lee et al. (
1993) (J Parasitol 79: 677-680) with permission.
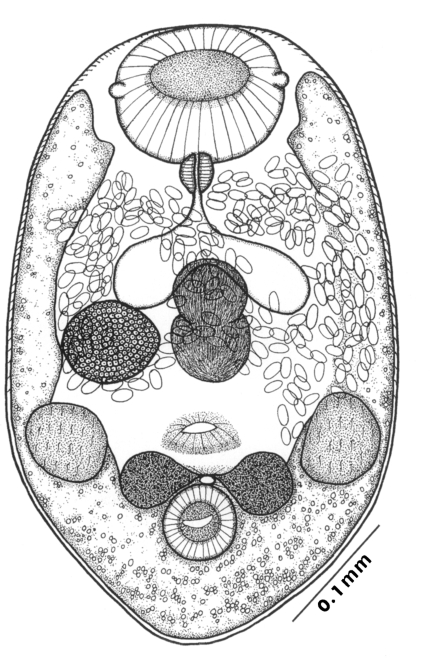
Fig. 2A fresh specimen of
Gymnophalloides seoi adult (ventral view, unstained) showing the ventral pit (arrowhead) and other structures. Reproduced from Lee et al. (
1994) (Am J Trop Med Hyg 51: 281-285) with permission.
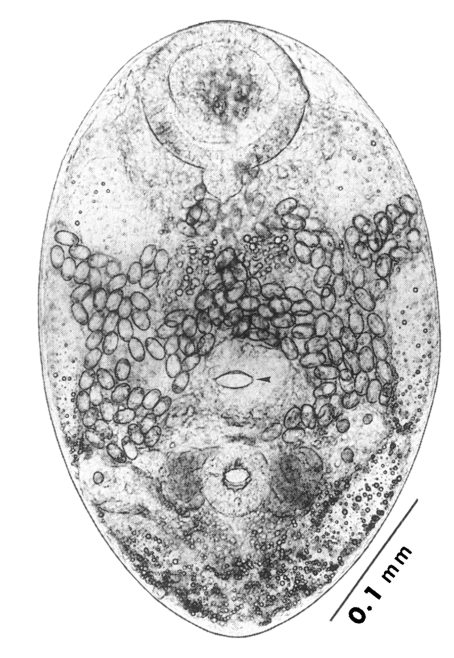
Fig. 3Diagrammatic drawing of the terminal reproductive system of
Gymnophalloides seoi, ventral view. lc, Laurer's canal; ga, genital atrium; gp, genital pore; pp, pars prostatica; ov, ovary; sv, seminal vesicle; t, testis; vt, vitellaria. Reproduced from Lee et al. (
1993) (J Parasitol 79: 677-680) with permission.
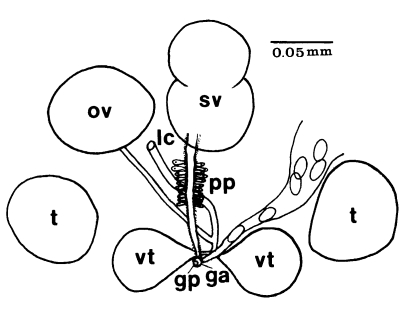
Fig. 4Longitudinal section of an adult Gymnophalloides seoi recovered from a human infection. os, oral sucker; ic, intestinal cecum, vt; vitellarium, vs, ventral sucker; gp, genital pore; vp, ventral pit. Bar: 0.05 mm.
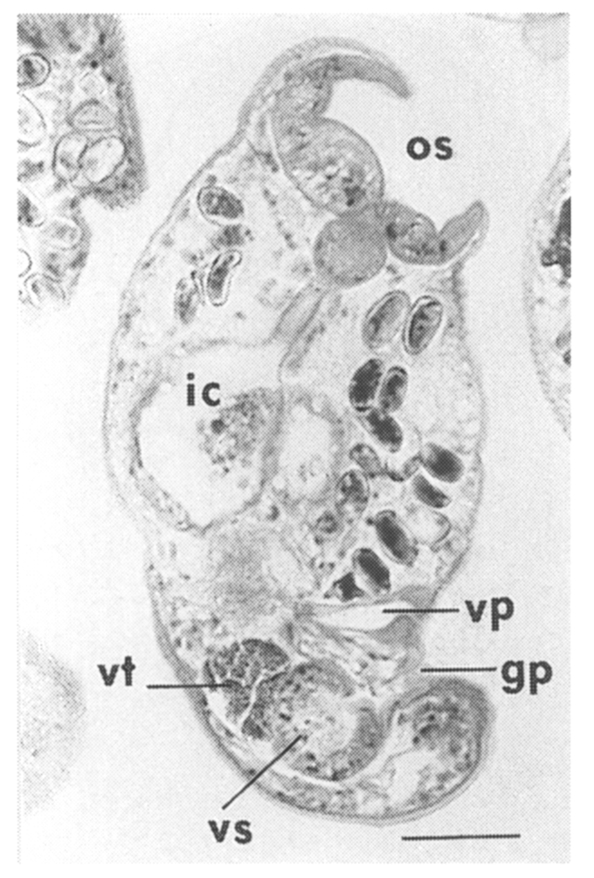
Fig. 5Eggs of
Gymnophalloides seoi in the feces of the first patient. A, High dry lens view, showing thin shell and prominent operculum; B, Oil immersion lens view. Reproduced from Lee et al. (
1993) (J Parasitol 79: 677-680) with permission.
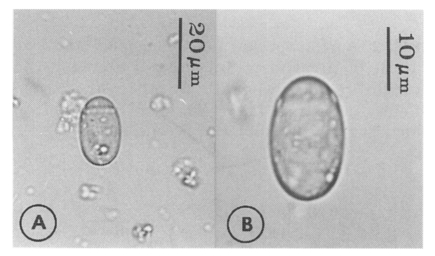
Fig. 6A metacercaria of Gymnophalloides seoi isolated from an oyster, not encysted. The large oral sucker, ventral pit (vp), and excretory bladder are distinctly observed.
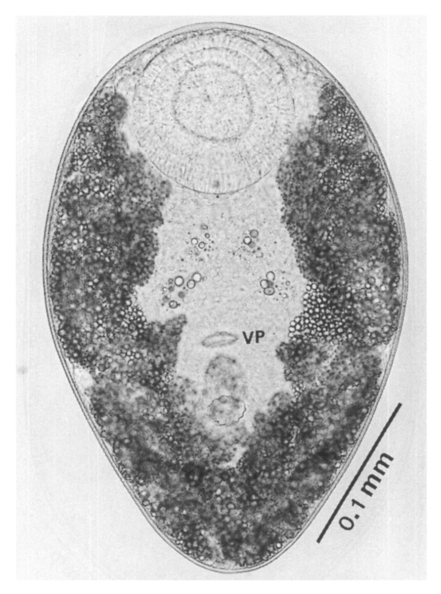
Figs. 7-10Scanning electron microscopic views of
Gymnophalloides seoi (metacercariae and adults). Reproduced from Choi et al. (
1995).
Fig. 7. A metacercaria of
G. seoi, ventral view. EP, excretory pore; OS, oral sucker; VL, ventrolateral lip; VP, ventral pit; VS, ventral sucker. Bar = 61.9 µm.
Fig. 8. A 3-dayold adult of
G. seoi, ventral view, showing the prominent oral and ventral suckers, and the ventral pit. Bar = 60.0 µm.
Fig. 9. Posterior portion of a
G. seoi metacercaria, ventral view, showing the relationships of the ventral pit (VP), genital pore (GP) and ventral sucker (VS). Bar = 7.4 µm.
Fig. 10. Dorsal tegument of a
G. seoi adult, showing numerous 8-10 pointed spines and a type I sensory papilla, near the anterior one-third of the body. Bar = 1.0 µm.
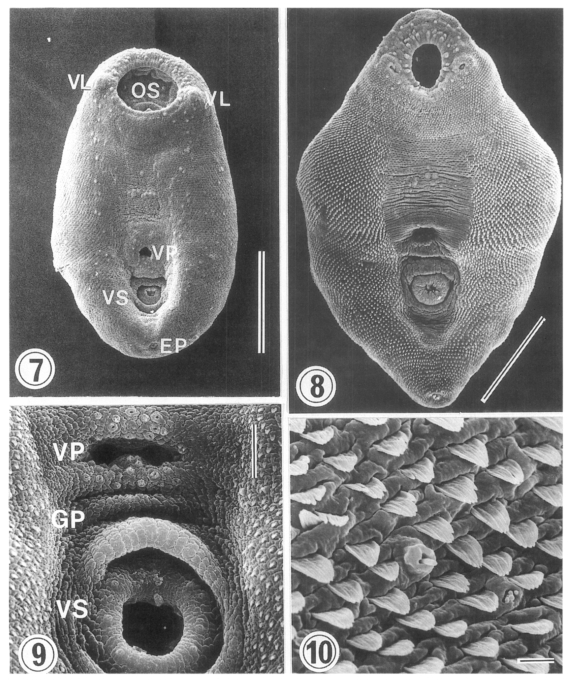
Figs. 11-14Transmission electron micrographs of the tegumental layer of
G. seoi (metacercariae and adults). Reproduced from Seo et al. (
1995).
Fig. 11. Tegumental layer of a metacercaria, showing the spines, basement membrane, circular and longitudinal muscle bundles, and nucleus (N) of a tegumental cell. ×6,000.
Fig. 12. Tegument of an adult fluke. Numerous small vacuoles (SV) and electron-dense granules (EDG) are seen in the syncytial layer. ×10,000.
Fig. 13. Ventral pit of an adult
G. seoi. Circular (C) and longitudinal (L) muscles are seen. The lumen (Lu) is leaf-like. Beneath the tegumental layer (TL) located a basement membrane (BM). Below it, circular (C) and longitudinal (L) muscles are seen. ×5,000.
Fig. 14. Magnification of the ventral pit, showing many mitochondria (arrows) and microtubules (arrowheads) in the tegumental layer. In a longitudinal muscle (L) between two circular muscles (C), a pair of mitochondria (M) are seen. ×20,000.
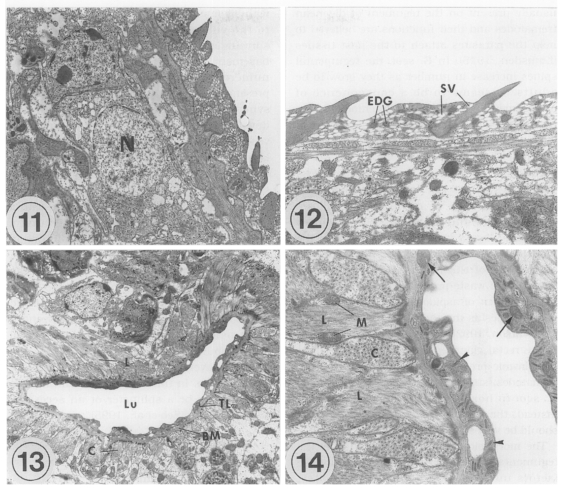
Figs. 15-16Oysters
Crassostrea gigas naturally infected with the metacercariae of
Gymnophalloides seoi, collected from Aphae-do, Shinangun. Reproduced from Lee et al. (
1995a).
Fig. 15. External view of the oysters.
Fig. 16. Internal view of an oyster, showing grouped metacercariae of
G. seoi (arrows) on the mantle of the oyster. Scale bar: 1.5 cm.
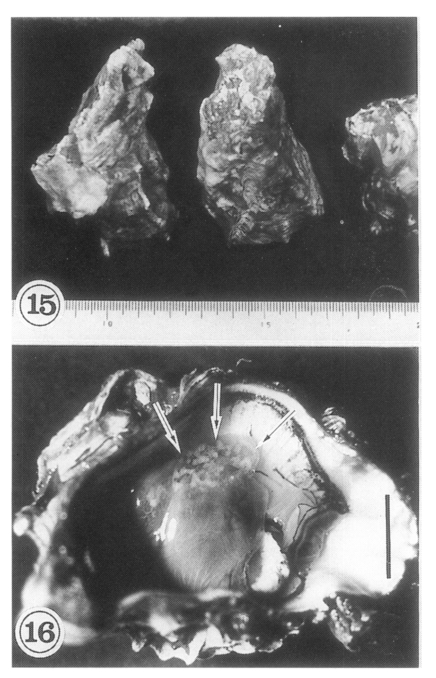
Figs. 17-20Metacercariae of
Gymnophalloides seoi attached on the mantle of the oyster. Reproduced from Lee et al. (
1995a).
Fig. 17. Magnification of
Fig. 16, showing numerous unencysted metacercariae of
G. seoi (arrows) on the surface of the oyster. Scale bar: 2 mm.
Fig. 18. A group of metacercariae attached on the mantle of an oyster as observed by a scanning electron microscope. Bar = 179 µm.
Fig. 19. Section of the mantle of an infected oyster. Some of them are sucking the extrapallial epithelia of the oyster with their oral sucker. ×100.
Fig. 20. Magnification of a metacercaria in Fig. 19, showing the oral sucker (OS) sucking the epithelial tissue of the oyster, ventral sucker (VS), ventral pit (VP), excretory bladder, and cecum (C). ×400.
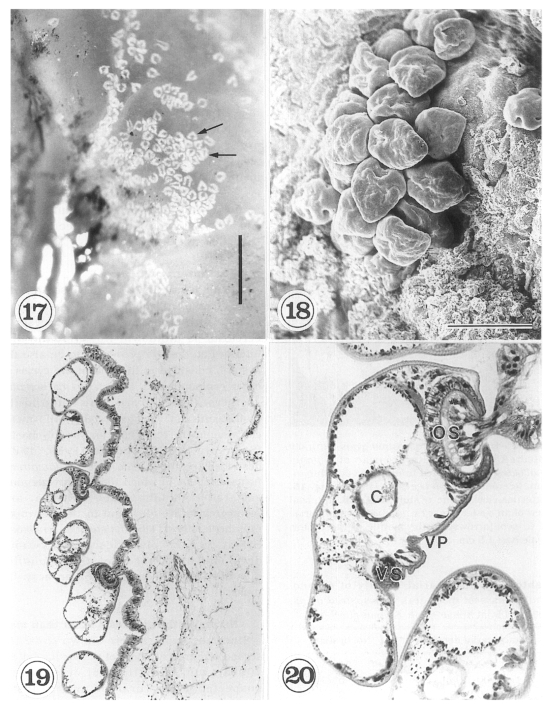
Fig. 21The Palearctic oystercatcher Haematopus ostralegus, a natural definitive host for Gymnophalloides seoi (Courtesy of Prof. Moo-Boo Yoon, Department of Biology, Kyunghee University, Seoul, Korea).

Figs. 22-23
Fig. 22. Section of the proximal jejunum of a C3H mouse infected with 300 metacercariae of Gymnophalloides seoi, day 5 post-infection (PI). Note the remarkable goblet cell (arrows) hyperplasia along the epithelial layer of the villi, inflammatory reactions in the crypts, and increased secretory granules in paneth cells of the crypts. ×100. Fig. 23. Middle jejunum of a mouse infected with G. seoi, day 5 PI. An adult fluke (arrows) is characteristically seen pinching the epithelial layer of a villus with its oral sucker. ×400.
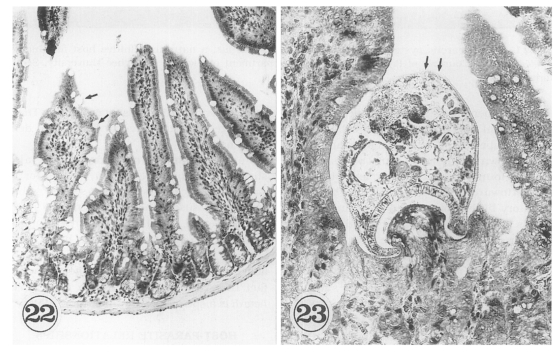
Fig. 24In situ postures of Gymnophalloides seoi adult flukes in the middle jejunum of an immunosuppressed C57BL/6 mouse, day 7 PI. The flukes are pinching and sucking the root of the host villi, and displacement as well as complete loss of the infected villi are seen nearby the worms. ×200.

Fig. 25Growth and development of
Gymnophalloides seoi in immunosuppressed C3H/HeN mice. A, metacercarial stage (unencysted); B, 1-day old worm; C, 3-day old worm; D, 5-day old worm; E, 7-day old worm; F, 14-day old worm. Reproduced from Chai et al. (
1999).
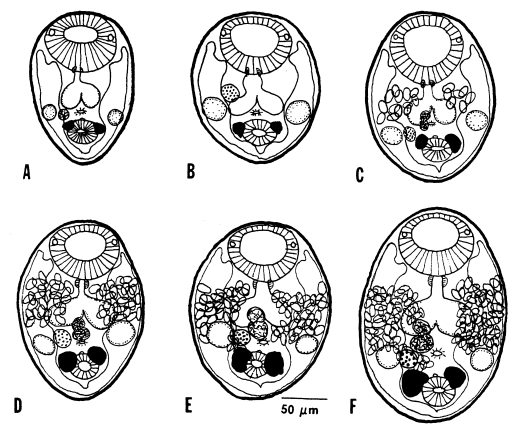
Fig. 26Number of uterine eggs of
Gymnophalloides seoi recovered from immunocompetent and immunosuppressed C3H/HeN mice. No worms were recovered from immunocompetent mice at days 14 and 21 PI. Significant difference was noted between worms from two groups of mice on day 5 and day 7 (P < 0.05). Reproduced from Chai et al. (
1999).

Fig. 27Map showing the geographical distribution of humans and oysters infected with
G. seoi. Data were obtained from Lee et al. (
19941),
1995b2),
19963)), Sohn et al. (
1998)
4), Lee et al. (
1999)
5), and Chai et al. (
19976),
19987),
2001a8)). Numbers represent the reference for each area (superscript).
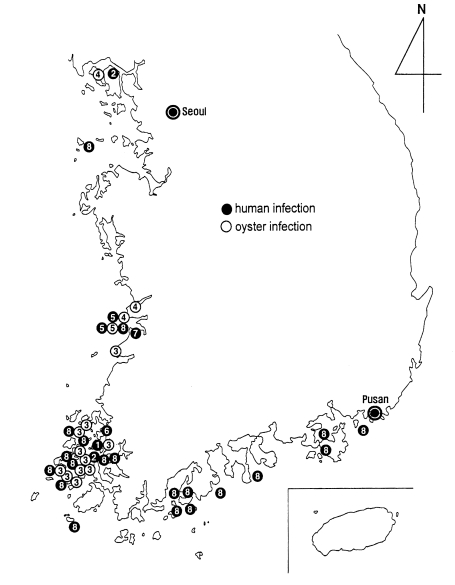
Table 1.Key characters
a) of the family Gymnophallidae Morozov, 1955
Table 1.
|
Genusb)
|
Gymnophallus Odhner, 1900 |
Gymnophalloides Fujita, 1925 |
Parvatrema Cable, 1953 |
Meiogymnophallus Ching, 1965 |
Lacunovermis Ching, 1965 |
|
Character |
|
Type species |
deliciosus
|
tokiensis
|
borinquenae
|
affinis
|
macomae
|
|
Ventral pit |
— |
+ |
— |
— |
+ |
|
Lateral papillae of oral sucker |
— |
+ |
+ |
+ |
+ |
|
Cecal pockets |
— |
— |
— |
— |
— |
|
Seminal vesicle |
Bi-, tri-partite |
Bipartite |
Undivided |
Undivided |
Undivided |
|
Prostatic duct |
Elongate |
Elongate |
Oval |
Oval |
Oval |
|
Genital pore, papillae |
Small, absent |
Small, absent |
Wide, present |
Small, present |
Wide, present |
|
Vitellaria |
Follicular |
Compact |
Compact |
Compact, lobed |
Compact |
|
Excretory vesicle |
Y shaped |
Y shaped |
V shaped |
V shaped |
V shaped |
|
Uterus extent |
Fore, hindbody |
Forebody |
Fore, hindbody |
Fore, hindbody |
Hindbody |
Table 2.Metacercarial density of
Gymnophalloides seoi in oysters
Crassostrea gigas, collected from Aphae-myon, Shinan-gun
a)
Table 2.
|
Metacercarial densityb)
|
No. oysters |
|
1-999 |
41 |
|
1,000-1,999 |
5 |
|
2,000-2,999 |
2 |
|
3,000-3,999 |
0 |
|
4,000-4,999 |
2 |
|
Total |
50 |
Table 3.Effects of immunosuppression on the recovery of Gymnophalloides seoi from C3H/HeN mice
Table 3.
|
Group and duration of Immunosuppression |
No. of micea) used |
Worm recovery rate (%)b)
|
|
Mean ± SD |
Range |
|
Immunocompetent mice |
5 |
11.8 ± 8.3 |
2.0 – 22.0 |
|
Immunosuppressed micec)
|
|
|
|
|
For 7 days |
6 |
27.8 ± 16.5 |
9.0 – 55.0 |
|
For 14 days |
6 |
33.8 ± 18.4 |
15.0 – 61.0 |
|
For 21 days |
6 |
67.5 ± 14.3 |
44.0 – 84.0 |
Table 4.Comparison of the status of infection with Gymnophalloides seoi among the people in a small village on Aphae-do, Shinan-gun
Table 4.
|
1994a)
|
1997b)
|
2000b)
|
|
Fecal examination |
|
|
|
|
No. of people examined |
98 |
94 |
107 |
|
No. of G. seoi egg positive cases (%) |
48 (49.0) |
67 (71.3) |
77 (72.0) |
|
Egg counts in E.P.G. |
|
|
|
|
No. of cases examined |
N.E.c)
|
63 |
65 |
|
Total E.P.G. |
— |
63,928 |
22,962 |
|
Mean E.P.G. |
— |
1,015 |
353 |
|
Worm collection |
|
|
|
|
No. of cases examined |
15 |
31 |
N.E |
|
No. of specimens collected |
49,896 |
320,677 |
— |
|
Mean no. of worms/case |
3,326 |
10,344 |
— |
|
Range of no. of worms/case |
106-26,373 |
94-69,125 |
— |