Abstract
The antigen location of Cryptosporidium parvum, which stimulates antibody formation in humans and animals, was investigated using infected human sera. Immuno-electron microscopy revealed that antigenicity-inducing humoral immunity was located at various developmental stages of parasites, including asexual, sexual stages, and oocysts. The amount of antigen-stimulating IgG antibodies was particularly high on the oocyst wall. The sporozoite surface was shown to give stimulation on IgG and IgM antibody formation. Trophozoites implicated the lowest antigenicity to humoral immunity, both IgG and IgM, by showing the least amount of gold labeling. Immunogold labeling also provided clues that antigens were presented to the host-cell cytoplasm via feeder organelles and host-parasite junctions.
-
Key words: Cryptosporidium parvum, cryptosporidiosis, immuno-electron microscopy, antigenicity, human serum
Cryptosporidium parvum (Apicomplexa), the causative agent for mammalian cryptosporidiosis, is an obligate intracellular parasite that develops in the brush border of epithelial cells in the digestive tract of numerous mammalian species, including humans [
1]. In the Republic of Korea, epidemiological studies on human cryptosporidiosis showed prevalence rates of 1-11% for immunocompetent inhabitants [
2-
5] and 11% for HIV-infected patients [
6] infected with
C. parvum.
A detailed description of the ultrastructure of various
Cryptosporidium life-cycle stages was published in previous studies [
7,
8], but little is known about the antigenic location according to different life-cycle stages. This study investigated the ultrastructural location of antigenicity of
C. parvum, which stimulates humoral immunity in humans.
The small intestines of C. parvum-infected (KKU isolate) female C57BL6/J mice were dissected out after 5 days of infection and fixed in 2% paraformaldehyde and 0.4% glutaraldehyde for approximately 2 hr at room temperature. Fixed tissues were washed with 0.1 M phosphate buffer (pH 7.2) and dehydrated through a 30-95% graded alcohol series. The dehydrated tissues were embedded in gold resin (London Resin, London, UK) and polymerized at -20℃ for 24 hr under UV illumination. Ultrathin sectioning was performed at a thickness of 90 nm, and sections were mounted on nickel grids. Human sera were obtained from C. parvum-infected patients who were diagnosed through fecal examinations. Negative control sera were collected from 10 infants without any relevant history of diarrhea. IgG and IgM titers of the sera checked by ELISA were in the range of above mean ± 2SD of negative control sera.
The immunogold labeling procedure followed the methods described by Yu and Chai [
9]. Tissue sections were incubated in PBS-milk-Tween (PMT; 3% skim milk and 0.01% Tween-20 in PBS) for 10 min and exposed to positive human sera diluted with PMT for 2 hr at room temperature. The sections were washed thoroughly with PBS-BSA-Tween (1% BSA, 0.01% Tween-20 in PBS) and incubated overnight with 5-nm-gold-conjugated goat anti-human IgG or IgM antibodies (Sigma, St. Louis, Missouri, USA) at 4℃. Silver enhancement was performed with a commercial kit (Amersham Biosciences, Uppsala, Sweden) followed by staining with uranyl acetate and lead citrate. The stained sections were examined under a transmission electron microscope (Hitachi, H-7650, Tokyo, Japan).
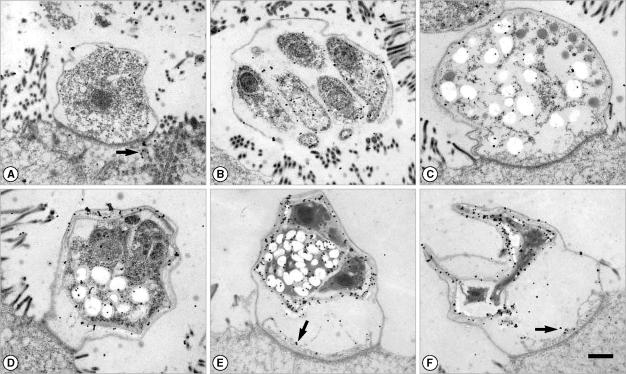
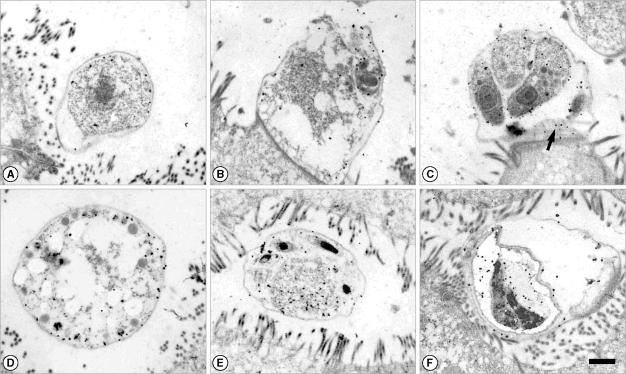
It was found that the amount of gold labeling in trophozoites was the lowest among the developmental stages (
Figs. 1A,
2A). Gold particles were labeled at the apical region and surface of merozoites by IgG and IgM antibodies (
Figs. 1B,
2B, C). Sexual stages such as macro- and microgametocytes were also labeled by gold particles for both IgG and IgM antibodies (
Figs. 1C,
2D, E). Wall-forming bodies of macrogametocytes were not labeled by antibodies (
Figs. 1C,
2D). In microgametocytes, the residuum was mainly labeled by IgM antibody (
Fig. 2E). The locality of IgG antibodies on microgametocytes was not observed. Oocysts showed strong antigenicity (
Figs. 1D-F,
2F). The oocyst wall of parasite origin and residuum in oocysts were heavily labeled by IgG antibodies (
Fig. 1D-F). However, the oocyst wall was not labeled by IgM antibodies (
Fig. 2F). In addition, both IgG and IgM antibodies were labeled at feeder organelles and the host-cell cytoplasm under host-parasite junctions (
Figs. 1A, E, F,
2C).
Pellicles of both sporozoites and merozoites of
C. parvum are already known to have strong antigenicity. Major antigens such as 23 kDa and 15 kDa have been localized on the surface plasma membranes of sporozoites and merozoites [
10,
11]. The pellicles around the anterior end of both sporozoites and merozoites exhibited specific labeling with antisera against
C. parvum [
10]. The results of our study suggest that the degree of antigenicity is lower for trophozoites than for other developmental stages, and this will be probably helpful for the successful initiation of infection. We also found that the membrane surface of merozoites was labeled by human sera having a high titer of IgG or IgM antibodies that also reacted with the 27 kDa antigen by western blot (data not shown). This finding was consistent with that of a previous study regarding a 23 kDa antigen [
10], notwithstanding the small difference in antigen size between the 2 studies.
We also found that sexual stages and oocysts exhibited some antigenicity that induced a humoral immune reaction in humans. It was interesting to note that the oocyst wall was labeled more by IgG than IgM antibodies. This finding is not consistent with an earlier study [
12]. Laxer et al. [
12] showed that the outer wall of oocysts and sporozoite membrane were heavily labeled by
Cryptosporidium-infected human IgM as well as IgG antibodies, but the nature of antibody labeling on other developmental stages was not described. The discrepancy of the results between this study and the previous one [
12] is difficult to explain. However, there is difference in the age of patients between the 2 studies whose IgM sera were used. The patient was a 34-year-old male in this study, whereas the patient was a 1-year-old male in the study of Laxer et al. [
12]. Previously, it was known that older adults have impaired immune responses when compared with young adults, as they have both decreased numbers of antigen-specific immune B-cells and decreased potency on a per cell basis [
13]. Specific antigen stimulating specific immune B-cells could possibly be different with maturation of the immune system according to aging.
C. parvum antigens localized at feeder organelles and the host-cell cytoplasm under host-parasite junction could imply that antigens of
C. parvum are allowed to pass between the parasite and host cell via such junctions. Little is known about the function of the interface between
Cryptosporidium and host cell cytoplasm. It has been suggested that it plays a role to modulate molecular interchange with the host or protect the parasite from host cell defenses [
14]. Further studies on the functional details of the host-parasite junction are needed.
In conclusion, we have found that the C. parvum oocyst wall reveal the highest degree of antigenicity for eliciting humoral immune reactions, especially IgG antibody production, in humans, whereas trophozoites has the lowest antigenicity among the developmental stages.
ACKNOWLEDGEMENTS
This study was supported by the program of the Basic Atomic Energy Research Institute (BAERI), which is a part of the Nuclear R&D Programs funded by the Ministry of Science and Technology (MOST) of Korea in 2008 and by the Second-Phase of the BK (Brain Korea) 21 Project, Ministry of Education, Science and Technology.
References
Fig. 1Immunogold labeling with a human serum which showed a high IgG titer to Cryptosporidium parvum crude antigens by ELISA. The patient was also diagnosed by fecal examination. (A) Trophozoite. Gold particles were labeled at both the outer pellicle and the host cell cytoplasm (arrow). (B) Merozoites in a mature meront. Gold particles were labeled at the merozoite surface and the apical region. (C) Macrogametocyte. (D) Immature oocyst with 2 sporozoites. (E, F) Oocysts with 2 sporozoites. Gold particles were heavily labeled at the oocyst wall and the residuum. The feeder organelle was also labeled (arrow). Bar = 1 µm.
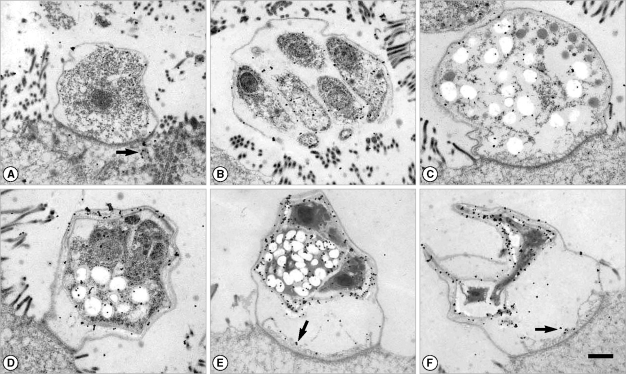
Fig. 2Immunogold labeling with a IgM-positive human serum to C. parvum crude antigens by ELISA. The patient was also diagnosed by fecal examination. (A) Trophozoite. (B) Immature meront. (C) Merozoites in a mature meront. Gold particles were labeled at the surface membrane. The feeder organelle was also labeled (arrow). (D) Macrogametocyte. (E) Microgametocyte. Gold particles were labeled at the pellicle and the residual body. (F) Oocyst with 2 mature sporozoites. Bar = 1 µm.

Citations
Citations to this article as recorded by

- Cryptostatin, a chagasin-family cysteine protease inhibitor ofCryptosporidium parvum
J.-M. KANG, H.-L. JU, J.-R. YU, W.-M. SOHN, B.-K. NA
Parasitology.2012; 139(8): 1029. CrossRef