Abstract
A family of calcium-dependent protein kinases (CDPKs) is a unique enzyme which plays crucial roles in intracellular calcium signaling in plants, algae, and protozoa. CDPKs of malaria parasites are known to be key regulators for stage-specific cellular responses to calcium, a widespread secondary messenger that controls the progression of the parasite. In our study, we identified a gene encoding Plasmodium vivax CDPK4 (PvCDPK4) and characterized its molecular property and cellular localization. PvCDPK4 was a typical CDPK which had well-conserved N-terminal kinase domain and C-terminal calmodulin-like structure with 4 EF hand motifs for calcium-binding. The recombinant protein of EF hand domain of PvCDPK4 was expressed in E. coli and a 34 kDa product was obtained. Immunofluorescence assay by confocal laser microscopy revealed that the protein was expressed at the mature schizont of P. vivax. The expression of PvCDPK4-EF in schizont suggests that it may participate in the proliferation or egress process in the life cycle of this parasite.
-
Key words: Plasmodium vivax, calcium-dependent protein kinase, schizont, EF-hand motif
Malaria, caused by the protozoan parasites,
Plasmodium spp., is one of the most important infectious diseases that lead to high morbidity and mortality in most of the tropical and subtropical areas of the world [
1]. Many efforts have been done to control and prevent malaria, but the global problems on malaria appear to be worsening [
2,
3]. The most important reason for this devastating situation is the emergence and spreading of drug resistance to currently affordable antimalarial drugs. Therefore, it is urgently needed to identify and characterize new potential targets for development of new antimalarial drugs.
A family of calcium-dependent protein kinases (CDPKs) is unique enzymes that are found in plants, green algae, ciliates, and apicomplexan parasites [
4]. In the
Plasmodium spp., members of the CDPK family are likely to be the key regulators that mediate stage-specific cellular responses to calcium, a widespread secondary messenger that controls the progression of the parasite through its complex life cycles [
5]. Moreover, CDPKs of the rodent malaria parasites, such as
Plasmodium berghei and
Plasmodium yoelii are known to be essential for parasite transmission to the mosquito [
6]. Due to the absence of CDPKs in mammals, including human, they are considered as promising target molecules for antimalarial drug development [
7,
8]. In this study, we identified a gene encoding
Plasmodium vivax CDPK4 (PvCDPK4) and characterized its molecular property and cellular localization of PvCDPK4 calmodulin region (EF-hand).
Blood samples infected with
P. vivax were collected from indigenous malaria patients in South Korea. The parasite DNA was extracted from the whole blood using Nucleospin® blood kit (MACHEREY-NAGEL, Düren, Germany). By data-mining the recently updated
P. vivax genome sequence database (PlasmoDB,
http://plasmodb.org), we identified a gene encoding PvCDPK4 (Gene ID: PVX_000555). The full-length gene of PvCDPK4 was amplified by PCR using the primers, 5'-ATGGGCCAAGAAATCTCCACGCCGAACGAC-3'and 5'-TTAATAGTTGCACAGCTTGACGAGCATGTC-3'with
Taq DNA polymerase (Takara, Otsu, Japan) and
P. vivax genomic DNA under following conditions: 95℃ for 5 min, 30 cycles of 95℃ for 1 min, 55℃ for 1 min and 72℃ for 1 min 30 sec, and 72℃ for 10 min for final extension. The resulting PCR product was purified from the gel using the Nucleospin extraction kit (MACHEREY-NAGEL), ligated into pCR2.1-TOPO Cloning Vector (Invitrogen, Carlsbad, California, USA) and then transformed into
Escherichia coli TOP10 (Invitrogen). The plasmid DNA was extracted by Nucleospin plasmid kit (MACHEREY-NAGEL) and the nucleotide sequence of the insert was analyzed by automatic sequencing. The genomic sequence, transcript sequences and amino acid sequences were extracted from the PlasmoDB 7.0 released [
9]. The Est2genome [
10] was used to align the transcript sequences with the genomic DNA sequence. We also searched for potential domains and motives on the coding sequences using the SWISS-MODEL workspace [
11]. Then, the EF hand motif of PvCDPK4 was amplified by PCR using primers, PvCDPK-EF-F (5'-GGATCCATGGGGTCCAAACTCACAACC-3') and PvCDPK-EF-R (5'-AAGCTTTCGCCGTCGTTGTTCTGGT-3'), under following conditions: 95 ℃ for 5 min, 30 cycles of 94℃ for 1 min, 67℃ for 1 min and 72℃ for 1 min 30 sec, and 72℃ for 10 min for final extension. The amplified PCR product was ligated into pET28a expression vector (Merck, Darmstadt, Germany) and transformed into
E. coli BL21 (DE3). Selected clones were grown and induced with 0.5 mM isopropyl-1-thio-β-D-galactopyranoside (IPTG). The recombinant protein was purified by nickel-nitrilotriacetic acid (Ni-NTA) chromatography (Qiagen) and analyzed by SDSPAGE. The specific antiserum against recombinant PvCDPK4-EF (anti-PvCDPK4-EF) was produced by immunizing BALB/c mice with the purified protein (50 µg) 3 times with 2 weeks intervals. The specificity of anti-PvCDPK4-EF was confirmed by immunoblot analysis and used in this study.
To determine the expression and confirm localization of PvCDPK4-EF in erythrocytic stage of
P. vivax, indirect fluorescence assay (IFA) by confocal laser scanning microscopy was performed as previously described with some modification [
12]. The
P. vivax-infected blood smears were fixed with a 50% acetone-50% methanol for 10 min at -20℃ and stored at -20℃ until use. The slides blocked with 5% skim milk for 30 min at 37℃ and washed with PBS. The slides were incubated for 30 min at 37℃ with 1:100 diluted anti-PvCDPK4-EF in PBS and washed 3 times with PBS. The slides were then incubated for 30 min at 37℃ with Alexa-Fluor 488-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, Oregon, USA). After washing 3 times with PBS, the slides were incubated with 25 µg of propidium iodide (PI) (Molecular Probes) for 10 min at 37℃. They were mounted with 50% glycerol in PBS and observed under a confocal laser scanning microscopy (TCS NT, Leica, Germany).
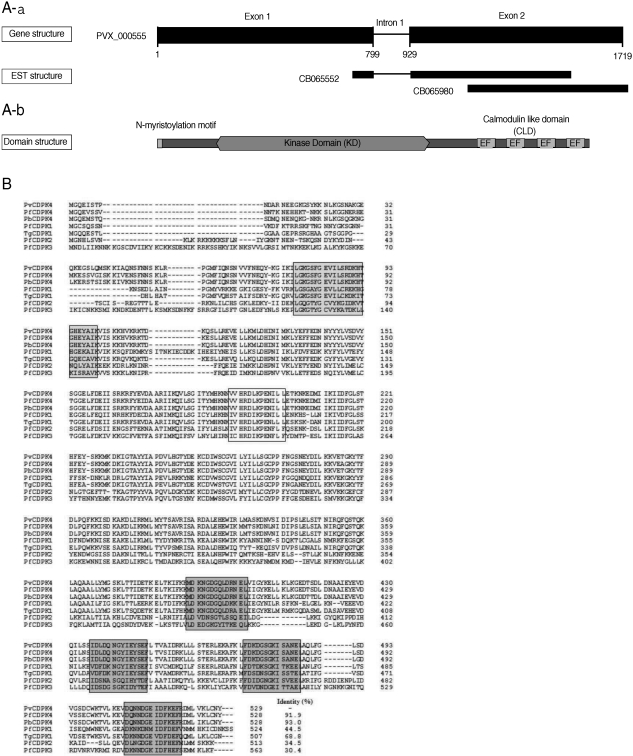
The gene encoding PvCDPK4 was consisted with 1,754 bp and contained an intron region (801-964 bp) in the middle of the sequence. We also analyze the gene structure of PvCDPK sequence in GenBank (PVX_000555) (
Fig. 1A). It was slightly different with our sequence as shown in
Fig 1A. Sequence analysis revealed that the PvCDPK4 gene contains a typical kinase domain (KD) and a calmodulin-like structure with 4 EF hand motifs that required for calcium-binding at C-terminus (
Fig. 1A). Multiple sequence analysis of the deduced amino acid sequence of PvCDPK4 with other presently known plasmodial CDPKs revealed that PvCDPK4 showed the highest sequence identity to PbCDPK4 (93.0%) and PfCDPK4 (91.9%), but less than 50% to PfCDPK1 (44.5%), PfCDPK2 (34.5%) and PfCDPK3 (30.4%) (
Fig. 1B).
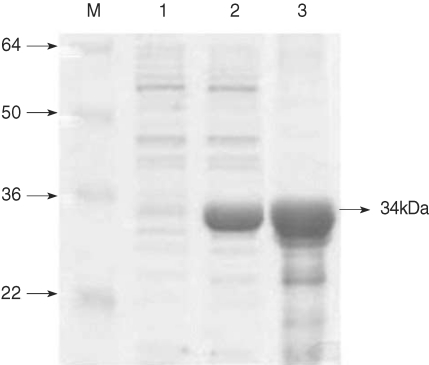
The recombinant PvCDPK4-EF was expressed as soluble form in
E. coli with the expected molecular weight of 34 kDa (
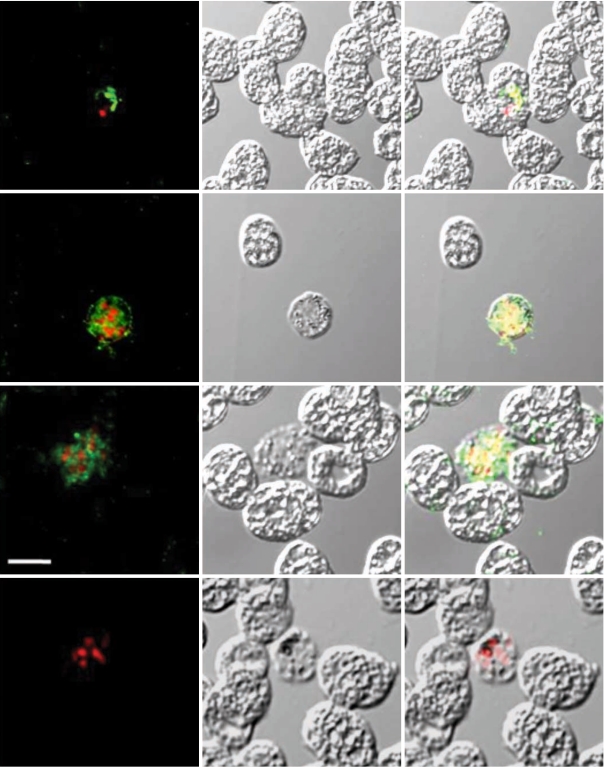
Fig. 2). In the IFA result to determine the expression pattern of PvCDPK4 in the erythrocytic stage of
P. vivax, interestingly, strong positive signals were identified in the schizont stage of the parasite, but not in other erythrocytic stages, including rings and trophozites (
Fig. 3, Panels II and III). Meanwhile, the negative control sera (Panel IV) did not show any reactions. The feature of PvCDPK4 specifically expressed in the schizont stage was similar to that of PfCDPK1 [
13]. PfCDPK1 is exported from schizonts to the parasitophorous vacuole membrane (PVM) of
Plasmodium falciparum, which would require the transfer of the protein across the parasite plasma membrane followed by insertion into the PVM [
13]. PfCDPK1 and PfCDPK4 belong to the same class in phylogenic analyses of calcium-dependent kinases in apicomplexans [
5]. This might support the similar pattern of localization of PfCDPK1 [
13] and PvCDPK4. It was also because we have analyzed EF-hand region of PvCDPK4 only. To report the evidences of the PvCDPK4 EF-hand expression, we searched for transcripts in the PlasmoDB datatbase [
9] and determined the structures of exon and intron of the PvCDPK4 gene using the alignment Est2genome program [
10]. In the case of PvCDPK4, we identified 2 transcripts, including EF-hand region that are likely to be partial fragments (
Fig. 1A-a). One transcript (CB065552) begins at the position +715 relative to the start codon for PvCDPK4 and has a single intron. The other (CB065980) begins at the position +808 and has no intron. Both of them possess the unique calmodulin-like domain (CLD) containing 4 EF hand domains in the coding region in spite of the partially expressed sequences (
Fig. 1A-a). Furthermore, another proof for the PvCDPK4 gene expression was found using the transcriptome profiling of
P. vivax described by Zbynek Bozdech [
14]. This also proves that the PvCDPK4 gene was highly expressed throughout the intraerythrocytic cycle of 3 distinct
P. vivax isolates. All these findings provide strong evidence that the calmodulin-like domain (CLD) of this gene is predominantly expressed in
P. vivax.
It has been known that calcium controls many critical events in the complex life cycles of apicomplexan parasites, including protein secretion, motility, and development, and, therefore, plasmodial CDPKs has recognized emerging as important targets for malaria parasite development [
5,
6]. Calcium released from intracellular stores also governs egress of tachyzoites of
Toxoplasma gondii [
15].
T. gondii calmodulin-like domain protein kinase isofrom 3 (TgCDPKif3) is activated by calcium in a dose-dependent manner and it mediates egress of the parasite through stimulation by calcium [
16]. A specific expression in the mature schizont stage of
P. vivax suggests that PvCDPK4 may participate in the development process and/or egress of the parasite. On the other hand, PfCDPK4 was detected on gametocytes [
17] and high through-put proteome analyses in the rodent species,
P. berghei and
P. yoelii, suggested that CDPK4 is abundantly expressed in both the gametocyte and ookinete stages, and that it is also detectable in asexual parasite stages [
6]. This fact suspected that PvCDPK4 may also serve in regulating the processes of sexual stage development, such as gametogenesis. However, we characterized only the EF-hand region of CDPK calmodulin-like domain in this study, thus it is difficult to say whether PvCDPK express in sexual stages or not. More complicated study would be necessary in the future to determine the physiological significance of PvCDPK4.
In conclusion, this is the first report on characterization of P. vivax CDPK. PvCDPK4-EF was specifically expressed in the schizont stage, which suggests that it is possibly related to the proliferation or invasion of the parasite in the parasite's life cycle. PvCDPK would represent antimalarial drug targets, leading to the development of more stage-specific drugs.
ACKNOWLEDGEMENTS
This study was supported by a grant from an intramural fund of the Korea National Institute of Health (KCDC 2006-N00238-00, 2007-N00320-00, 2008-N00410-00) and by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) No. 2010-0004043. Anti-MSP mouse monoclonal antibody was kindly provided by Dr. Chom-Kyu Chong, Bioland, Cheongwon Research Institute, Korea.
References
- 1. Arevalo-Herrera M, Herrera S. Plasmodium vivax malaria vaccine development. Mol Immunol 2001;38:443-455.
- 2. Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg 2001;64:1-11.
- 3. Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol 2001;17:593-597.
- 4. Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinases regulates Plasmodium ookinete access to the midgut epithelial cell. Mol Microbiol 2006;59:1175-1184.
- 5. Billker O, Lourido S, Sibley LD. Calcium dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe 2009;5:612-622.
- 6. Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 2004;117:503-514.
- 7. Farber PM, Graeser R, Franklin RM, Kappes B. Molecular cloning and characterization of a second calcium-dependent protein kinase of Plasmodium falciparum. Mol Biochem Parasitol 1997;87:211-216.
- 8. Harper JF, Harmon A. Plant, symbiosis and parasites: a calcium signaling connection. Nat Rev Mol Cell Biol 2005;6:555-566.
- 9. Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Miller JA, Nayak V, Pennington C, Pinney DF, Roos DS, Ross C, Stoeckert CJ Jr, Treatman C, Wang H. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res 2009;37:D539-D543.
- 10. Mott R. EST_GENOME: A program to align spliced DNA sequences to unspliced genomic DNA. Comput Appl Biosci 1997;13:477-478.
- 11. Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Res 2009;37:D387-D392.
- 12. Angel DI, Mongui A, Ardila J, Vanegas M, Patarroyo MA. The Plasmodium vivax Pv41 surface protein: identification and characterization. Biochem Biophys Res Commun 2008;377:1113-1117.
- 13. Green JL, Ress-Channer R, Howell SA, Martin SR, Knuepfer E, Taylor HM, Grainger M, Holder AA. The motor complex of Plasmodium falciparum: phosphoryation by a calcium-dependent protein kinase. J Biol Chem 2008;283:30980-30989.
- 14. Bozdech Z, Mok S, Hu G, Imwong M, Jaidee A, Russell B, Ginsburg H, Nosten F, Day NP, White NJ, Carlton JM, Preiser PR. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci USA 2008;105:16290-16295.
- 15. Moudy R, Manning TJ, Beckers CJ. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem 2001;276:41492-41501.
- 16. Sugi T, Kato K, Kobayashi K, Pandey K, Takemae H, Kurokawa H, Tohya Y, Akashi H. Molecular analyses of Toxoplasma gondii calmodulin-like domain protein kinase isoform 3. Parasitol Int 2009;58:416-423.
- 17. Ranjan R, Ahmed A, Gourinath S, Sharma P. Dissection of mechanisms involved in the regulation of Plasmodium falciparum calcium-dependent protein kinase 4. J Biol Chem 2009;284:15267-15276.
Fig. 1(A) Gene and domain structure of PvCDPK4. (a) The PvCDPK4 locus of P. vivax (CM000444: 475,102-476,845, PlasmoDB 7.0). The PvCDPK4 gene structure (nucleotides 1-1590) comprises 2 exons. The black boxes in the PvCDPK4 gene represent exons of CDS. There are 2 expressed sequence tags (ESTs) in the region of the PvCDPK4 locus. (b) The domain structure of the PvCDPK4. The PvCDPK4 contains a diverse variety of protein domains and common patterns can be identified as PfCDPK4. The calciumdependent protein kinase 4, encoded by PvCDPK4, is a 529 amino acid (aa) protein with 4 EF hand repeats known as calmodulin-like domain (CLD), which possesses Nterminal myristoylation motif and kinase domain (KD). (B) Sequence alignment of the deduced amino acid sequence of PvCDPK4 with other related proteins. The GenBank/EMBL database accession numbers are as follows: PfCDPK1, X67288; PfCDPK2, X99763; PfCDPK3, AF1060641; PfCDPK4, XM_001349042; PbCDPK4, AY555067; TgCDPK1, AF333958. Sequences were aligned with BioEdit ver 7.0 software (Tom Hall Isis Therapeutics, Isis Pharmaceuticals, California, USA). The numbers of amino acid residues are to the right of the sequences. Protein kinase ATP binding domain (grey box), serine/threonine kinase active site (open box), and 4 calcium binding EF-hand motifs (dark grey boxes).

Fig. 2Expression of PvCDPK4-EF. Expression and purification of the recombinant PvCDPK4-EF. The expression of recombinant protein was induced by adding IPTG to the final concentration of 0.5 mM and analyzed on SDS-PAGE followed by Coomassie blue staining. Lane 1, non-induced E. coli; Lane 2, IPTG-induced E. coli; Lane 3, Ni-NTA affinity purified recombinant protein. The recombinant PvCDPK4-EF protein was observed as approximately 34 kDa.

Fig. 3Indirect fluorescent assay (IFA). P. vivax-infected blood smears were probed with anti-rPvCDPK4-EF followed by nuclear staining with propidium iodide (PI) and observed with confocal scanning laser microscopy. FL, fluorescence microsocpe; DIC, differential interference contrast microscope; Merge, merged image of FP and DIC. Panel I (positive control), probed with anti-MSP mouse monoclonal antibody and PI; panel II and III, probed with anti-PvCDPK4-EF and PI; panel IV (negative control), probed with normal mouse serum and PI. Scale bars indicate as 5 µm.