Abstract
Acanthamoeba spp. commonly cause Acanthamoeba keratitis which is typically associated with the wear of contact lenses. Therefore, finding an economic, efficient, and safe therapy of natural origin is of outmost importance. This study examined the in vitro lethal potential of ethyl acetate and methanol extracts of Helianthemum lippii (L.) (sun roses) against Acanthamoeba castellanii cysts isolated from patients with amoebic keratitis. Both extracts proved to be potent as regard to their lethal effects on A. castellanii cysts with comparable results to chlorhexidine. The ethyl acetate was more promising with cumulative lethality. It showed a highly significant lethal percentage along the duration of treatment. The analysis of the more potent ethyl acetate extract revealed the presence of 2.96 mg/100 g of total phenolics, 0.289 mg/100 ml of total flavonoids and 37 mg/100 mg of total tannins which highlighted their phytomedicinal role.
-
Key words: Acanthamoeba castellanii, Helianthemum lippii, sun rose, ethyl acetate, methanol, keratitis
INTRODUCTION
Acanthamoeba is a group of single-celled free-living amoebae that are opportunistic pathogens of humans. There are 2 life cycle stages in this environmental amoeba, an infective trophozoite and a resilient cyst [
1]. Trophozoites live on a variety of bacteria, when environmental conditions become unfavorable; the organism encysts. In the cyst form, the amoeba is capable of surviving up to a year and is resistant to temperature and pH [
2].
Acanthamoeba spp. have been isolated from several habitats [
3], including soil, dust, air, natural and treated water, sea water, drinking water, bottled water, dental treatment units, dialysis units, eyewash stations, and contact lenses and lens cases [
4].
Acanthamoeba spp. commonly cause
Acanthamoeba keratitis [
5]. The first signs are inflammation with redness, epithelial defects and photophobia, edema, pain due to radial neuritis, epithelial loss, and stromal abscess formation with vision-threatening consequences [
3]. The most characteristic clinical feature is the presence of a ring-like stromal infiltrate, thought to correspond to the infiltrating inflammatory cells [
6]. It is typically associated with the use of contact lenses [
4] which seems to be the most important factor. Treatment of
Acanthamoeba keratitis regimen includes combination therapy which uses 2-3 biocides, such as biguanides, chlorhexidine, and polyhexamethylene biguanides, or in combination with diamidines which are effective in the treatment [
6]. Resistance to chemotherapeutic agents is probably the principal factor contributing to the increase in cases that may result in loss of vision.
In an effort to improve the therapy, medicinal plants are used as a source of new agents with high activity and low toxicity. Extracts and essential oils were effective in controlling the growth of a wide variety of microorganisms, including bacteria, parasites, yeasts, and filamentous fungi. Medicinal plants such as
Thymus [
7],
Salvia staminea [
8],
Ipomoea sp.,
Kaempferia galanga, Cananga odorata [
9],
Teucrium polium, Teucrium chamaedrys [
10],
Pastinaca armenea, Inula oculus-christi [
11],
Peucedanum species [
12],
Rubus chamaemorus, Pueraria lobata, Solidago virgaurea, Solidago graminifolia [
13],
Pterocaulon polystachyum [
14], and
Allium sativum [
15,
16],
Arachis hypogaea L
., Curcuma longa L
. and
Pancratium maritimum L [
17] extracts have proven to be effective growth inhibitors to
Acanthamoeba even more than the currently used therapy.
Cistaceae family is indigenous to the Mediterranean countries and comprises 200 species, including
Helianthemum (
H.) spp. (sun roses) which are cultivated as ornamentals [
18].
Helianthemum lippii (L.) (syn.
Helianthemum sessilflorum) methanol and CHCl
3 extracts were reported to exhibit significant analgesic and anti-inflammatory activities [
19]. Other species,
Helianthemum glomeratum, an endemic medicinal herb which has gained popularity with the name 'cenicilla', was used to treat abdominal pain, infectious diarrheal diseases, stomach pain, worm infections, and dysentery. Methanol extracts of the aerial parts of
H. glomeratum had antibacterial, antifungal, and antiprotozoal properties [
20,
21,
22]. In relation to phytochemical studies, 3 flavonoids (kaempferol, quercetin, and tiliroside) were isolated from the aerial parts, with anti-amoebic activity [
23]. Another phytochemical study done with
H. glomeratum roots, led to the identification of flavan-3-ol, (-)-epigallocatechin as the main anti-giardial and anti-amoebic compound [
24]. Although from this family many species have been studied, few reports could be traced on
H. lippii (L.).
Thus, the present study reports the isolation and anti-acanthamoebic activities of ethyl acetate and methanol extracts from H. lippii with comparative evaluation of their in vitro efficacy on Acanthamoeba castellanii cysts isolated from patients with amoebic keratitis.
MATERIALS AND METHODS
Chemicals and reagents
All the chemicals used were analytical grade. Quercitin and gallic acid were purchased from Across organics (Geel, Belguim). The n-hexane, ethyl acetate, methanol, DMSO, and chlorhexidine gluconate were obtained from Sigma and Roth (Strasbourg, France). UV spectra were recorded in methanol extract on a Shimadzu UV-1650 PC UV/Visible spectrophotometer; λ max (nm). Ready-made silica gel GF 254 plates, aluminum sheets for TLC (E. Merck, Darmstadt, Germany). The chromatograms were detected with a UV lamp at 254 and 365 nm.
Plants
The plant was collected from the northern part of Egypt, El Alameen, and kindly identified by senior botanist Mohamed Gibali. A voucher specimen no. 2009BUPD25 is available for inspection at the Department of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University, Egypt. The aerial parts of
H. lippii (L.) (
Fig. 1) (100 g) were exhaustively extracted, by maceration, with methanol extract at room temperature. The extract was then concentrated under reduced pressure to give a semisolid residue. After removal of the solvent, the residue was defatted with petroleum ether. The defatted portion was suspended in H
2O and partitioned into 2 portions; ethyl acetate extract and methanol extract. The 2 portions were subjected to the biological study.
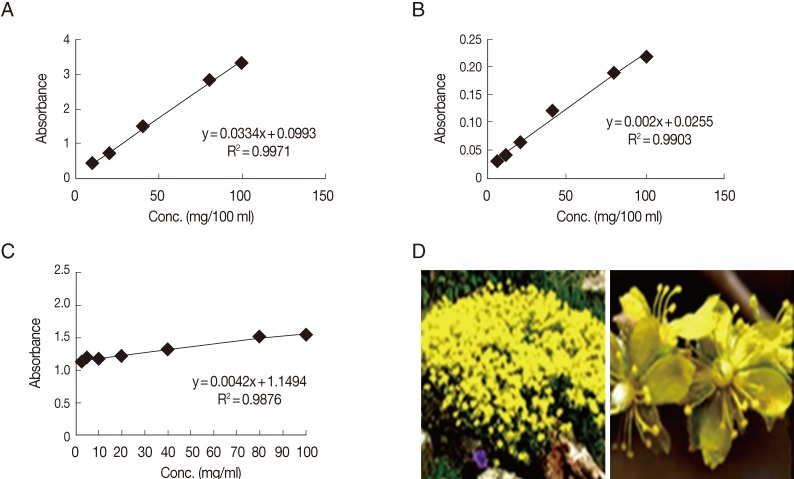
Based on the biological results, a further phytochemical screening of the ethyl acetate extract was performed spectrophotometrically. The dried ethyl acetate extract was tested for the presence of flavonoids, phenols, and tannins by giving intense yellow color with an alkali for flavonoids and by giving dark blue or green for phenols and tannins. The total phenolic content present in the ethyl acetate extract was estimated by the Folin-Ciocalteu method. A weighed amount (0.5 g) of the tested extract was mixed with 50 ml of 80% methanol for 20 min. After filtration of the extract, the filtrate was adjusted to 50 ml with methanol. Aliquot amounts of the individual extracts were used for determination of total phenolic contents. The experiments were carried out in duplicate. Different dilutions (20, 40, 60, 80, and 100 mg/ml) of gallic acid were used for drawing of the standard curve. An aliquot of 1 ml each of the tested extracts and gallic acid solutions were introduced into a volumetric flask (25 ml) containing distilled water (9 ml). One ml of the reagent Folin-Ciocalteu was added, followed by 10 ml of Na
2CO
3 (7%) after 5 min. The solution was mixed carefully and left at room temperature for 1.5 hr. A blank experiment was processed with distilled water. The absorbance was recorded at 750 nm colorimetrically. Results of total phenolic content, in each sample, expressed as mg/g of gallic acid equivalent was deduced from the standard curve [
25] (
Fig. 1).
Total flavonoid content of each treatment were determined using AlCl
3 colorimetric method. One gram of the ethyl acetate extract was macerated in 25 ml of 80% methanol solution for 24 hr in room temperature. The filtrate was adjusted to 25 ml with 95% methanol solution, and 1 ml of 2% AlCl
3 in methanol was added to 1 ml of the resulted filtrate. After 15 min, the absorption of the solution was measured using a spectrophotometer at 510 nm against AlCl
3 solution. The total flavonoids were determined using the calibration curve of quercitin, and the results are expressed as mg/100 ml [
26] (
Fig. 1).
One gram of the dried ethyl acetate extract was thoroughly extracted with 100 ml of distilled water for 1 hr, at room temperature, using a shaker. The filtrate was adjusted to volume with distilled water. An aliquot (10 ml) of the filtrate was transferred to the spectrophotometer tube and treated with 5 ml of 0.1 M FeCl
3 solution (dissolved in 0.1 N HCl containing 0.008 M potassium ferrocyanide). The absorbance of the color was measured, within 10 min, at 120 nm, and the blank solution used was prepared with distilled water. A standard solution of gallic acid was used for comparison. The tannin content was calculated in terms of gallic acid equivalent mg/100 mg dry weight [
27] (
Fig. 1).
Corneal scrapings were collected from keratitic patients attending the corneal outpatient clinic of the Research Institute of Ophthalmology (RIO), Giza, Egypt, where
Acanthamoeba isolation and testing were performed in the Diagnostic and Research Laboratory of Parasitic Diseases, Parasitology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt. The specimens were inoculated directly onto the surface of 1.5% non-nutrient agar (NNA) plates seeded with
Escherichia coli bacterial suspension and incubated in a humidified chamber at 30℃ [
28]. The presence of
Acanthamoeba could be seen by the clear tracks on the
E. coli lawn NNA produced by the feeding trophozoites of
Acanthamoeba. Examination of the agar plate surface for the presence of amoebic growth was carried out daily for up to 7 days with light and inverted microscopes using a ×40 objective.
Acanthamoeba was identified by the specific morphology of cysts and trophozoites. Subcultures were done after 2 weeks from positive cultures with confirmed amoebic growth by cutting a small square of agar using a sterile scalpel and placing it upside down on new NNA-
E. coli plates. The plates were incubated in humidified chambers at 30℃ and examined after 24 hr. Performing sub-culturing several times facilitated the isolation of
Acanthamoeba. Cysts were collected from 3-week cultures. The agar surfaces were flooded with 5 ml of PBS and were gently scraped with an inoculating loop. Cysts were harvested from the suspension by centrifugation at 350 g for 10 min. The supernatant was aspirated, and the sediment was washed twice in PBS in order to eliminate most of the bacteria. Cysts in the resultant suspension were counted with a hemocytometer, and the suspension was standardized to be 25×10
4/ml [
29].
In order to evaluate the in vitro amoebicidal activity of methanol extract and ethyl acetate extracts of
H. lippii on
Acanthamoeba cysts, amoebae were incubated with different concentrations of
H. lippii ethyl acetate extract and
H. lippii methanol extract, 500 (C1), 250 (C2), 125 (C3), 64 (C4), 32 (C5), 16 (C6), and 8 (C7) mg/ml, adjusted by dilution of each in dimethyl sulfoxide 1% (DMSO) to the specified concentration of the drug for different incubation periods (24, 48, and 72 hr). A 100-µl of the calibrated cyst suspension (2.5×10
5/ml) was inoculated into each well of a 96-well plate, left for 30 min to avoid disturbance of the adherence of amoebae onto the wells' surface. PBS solution was removed, and 100 µl of each concentration of the plant fractions was added into the wells. The plate was incubated at 30℃ for different incubation periods. In addition, non-treated control (negative control) containing only the parasite in PBS and drug control (positive control) containing the parasite plus 0.02% chlorhexidine gluconate (prepared from a solution 20% in H
2O CHX, C-9394; Sigma) were used. The effect of DMSO was excluded by using a well containing the parasite plus 1% DMSO. Trials were performed in triplicates. After each incubation period, 100 µl from each test and control well was transferred into 100 µl of 0.3% basic methylene blue media for counting the unstained (viable) and stained (non-viable) cysts by hemocytometer, 10 min after stain addition. Cultures containing non-viable cysts were inoculated onto NNA-
E. coli plate, incubated at 30℃ for an additional 72 hr, and examined to detect any viable cysts [
16].
The collected data was revised, coded, tabulated and introduced to a PC using Statistical package for Social Science (SPSS 15.0.1 for windows; SPSS Inc., Chicago, Illinois, USA). An informed consent was taken from all the patients after explaining the aim of the study. The study was approved by the research Ethics Committee, Faculty of Medicine, Ain Shams University, Egypt.
RESULTS
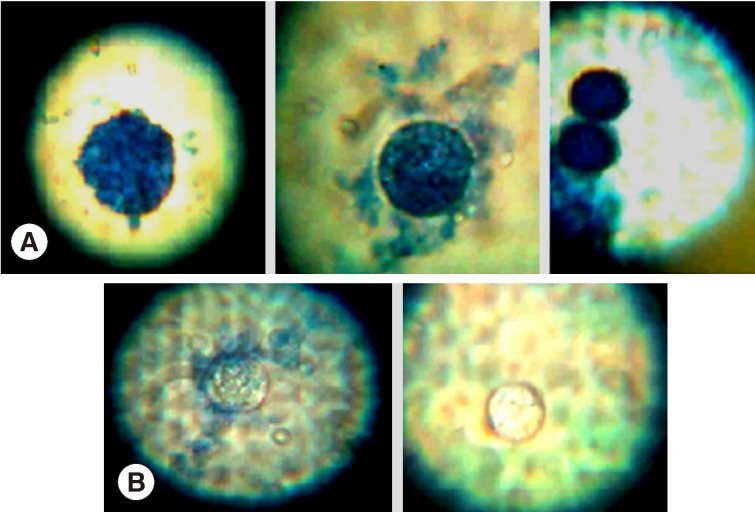
Light microscopic appearances of non-viable (stained) and viable cysts (unstained) after incubation with
H. lippii (L.) extracts are shown in (
Fig. 2). Ethyl acetate extract showed a highly significant lethal percentage along the duration of treatment in comparison to methanol extract which showed no significant difference as regard to the percentage of non-viable cysts along the duration of treatment (
Table 1). There was a significant difference between ethyl acetate extract and methanol extract regarding the percentage of non-viable cysts at days 1 and 2 and highly significant difference at day 3 (
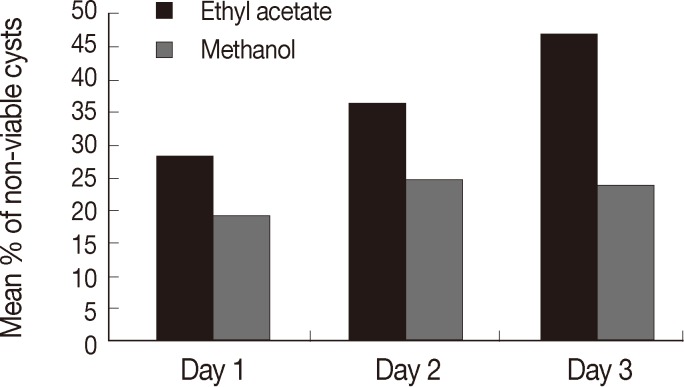
Table 1). Ethyl acetate showed higher percentage of non-viable cysts at different follow-up intervals, and the highest mean of non-viable cysts was reported in the 3rd day of incubation regarding all the drug concentrations (47.5±22.9) while the mean among methanol was highest at day 2 (25±12.1) (
Fig. 3).
There was a highly significant difference between ethyl acetate extract and negative control regarding the percentage of non-viable cysts at days 1, 2, and 3. Ethyl acetate extract showed higher percentage at different follow-up intervals, on the other hand, there was a highly significant difference between methanol extract and negative control (non-treated parasite) at days 1 and 2 only where methanol extract showed higher percentage of non-viable cysts. However, at day 3 no significant difference was detected (
Table 1).
There was a highly significant difference between ethyl acetate extract and positive control (chlorhexidine) in the percentage of non-viable cysts at day 1 and significant difference at day 2. Ethyl acetate extract showed higher percentage of non-viable cysts; however, at day 3 no significant difference was detected between both ethyl acetate extract and positive control. There was a highly significant difference between methanol extract and drug control in the percentage of non-viable cysts at days 1 and 3. Methanol extract showed higher percentage of non-viable cysts at day 1 (
Table 1).
Regarding the different concentrations, 500 (C1), 250 (C2), 125 (C3), 64 (C4), 32 (C5), 16 (C6), and 8 (C7) mg/ml, they all gave best results at the 3rd day of incubation using ethyl acetate extract; except for the fifth concentration (C5) which gave best results on the 1st day of incubation. Regarding methanol extract, there was no rule for the different concentrations in the different incubation periods. First and forth concentrations (C1, C4) gave the best results in the first day of incubation, while second, third, fifth, and seventh concentrations (C2, C3, C5, C7) showed their best at day 3 and sixth concentration (C6) gave its best results at day 2 (
Table 2).
Regarding the different concentrations of ethyl acetate extract and methanol extract as regard to the percentage of non-viable cysts at days 1, 2, and 3, there was a significant difference between the different concentrations of ethyl acetate extract at days 2 and 3, while non-significant difference was detected at day 1. There was a highly significant difference between the different concentrations of methanol extract at days 1 and 2, and significant difference at day 3 (ANOVA, post hoc test) (
Table 2). Based on these biological results, a further phytochemical screening of ethyl acetate extract, being more potent, was performed. The content of total phenolics, flavonoids, and tannins were calculated as 2.96, 0.289, and 37 mg/100 mg, respectively.
DISCUSSION
Acanthamoeba keratitis is a chronic infection and difficult to treat. Moreover, failure to control infection can lead to permanent loss of vision [
30]. Early diagnosis is crucial for the successful treatment of
Acanthamoeba infections [
31]. Flowering plants produces a variety of potent drugs. Globally, there is a raising trend to shift resources from allopathic to traditional health care systems [
32].
The
Helianthemum taxa used in folk medicine did not cluster in a unique section, being equally distributed in 2 out of the 4 sections analyzed. There was no clear relationship between the chemotype based on the polyphenolic composition and their taxonomical classification. However, the composition of the methanol and water extracts from the leaves of plants belonging to the
Helianthemum genus was strongly related to their medicinal uses. The
Helianthemum genus contains approximately 100 taxa. Some of them are important medicinal plants used in several countries for many different purposes. However, studies addressing the biological activities of many of these species or their phytochemistry are currently non-existent [
33]. The Cistaceae family has proved great phytomedicinal potency [
18], including species like
H. lippii [
19] and
H. glomeratum [
20,
21,
22]. Preliminary phytochemical screening of
H. lippii revealed the presence of flavonoids, tannins, glycosides, simple phenolics, free reducing sugars, and saponines [
19].
Our study aimed to evaluate the anti-acanthamoebic activities of H. lippii ethyl acetate and methanol extracts in vitro. In the present study, both extracts showed high potency in their lethal effects on A. castellanii cysts, and ethyl acetate was more promising with regard to its cumulative lethal effects.
In this study, the phytochemical screening of the ethyl acetate extract revealed a highest concentration of tannins which highlighted its lethal potency and opens a way to study the selective and synergetic action with the other fractions; total phenolics and flavonoids. It was proved previously that semi-purified polyphenolic fractions of
H. glomeratum were effective against bacterial isolates. Polyphenolics or the so-called vegetable tannins are widely distributed in medicinal plants. Recent advances in separation procedures have led to the isolation of a large number of this type of compound which has shown a variety of biological activities, such as antiviral, antioxidant, and anti-tumor. Polyphenols were fractionated to Po5 and epigallocatechin which proved to have strong anti-amoebic activities [
24]. Antibacterial and antifungal activities have also been reported [
34].
The polyphenolic profile was specific for each taxon. Whereas
Helianthemum alypoides,
Helianthemum cinereum, Helianthemum hirtum, Helianthemum asperum, and
Helianthemum marifolium were characterized by the presence of gallic acid, egallic derivatives, and ellagitannins; the polyphenolic profile of
Helianthemum apenninum, Helianthemum syriacum, and
Helianthemum polygonoides was mostly based on flavonoids.
Helianthemum cinereum,
Helianthemum alypoides, and
Helianthemum marifolium consistently presented the strongest radical scavenging activity and antimicrobial activities [
33].
Previously methanol extracts obtained from the aerial parts and the roots of
H. glomeratum were active against axenic
Entamoeba histolytica and
Giardia lamblia trophozoites in vitro. The flavonoids found in the aerial parts, tiliroside and kaempferol, were shown to be inhibitors of the growth of both parasites, kaempferol being the most active [
24].
The inhibitory concentrations of kaempferol and tiliroside in the leaves [
23] were similar to that of (ÿ)-epigallocatechin in the roots, it was suggested that these flavonoids participate in the activity of the plant. Epigallocatechin and epigallocatechin gallate were claimed to be promising anticancer agents [
35]. Overall, our findings confirmed that ethyl acetate and methanol extracts of
H. lippii had a lethal potential as acanthamoebicidal agents with more attention to ethyl acetate. In vivo model is still needed to detect whether these extracts are good enough to control and eradicate this life-threatening pathogen. In addition, further analysis is essential to validate the chemical composition and possible active compounds which may serve as a new source of chemotherapeutic agents for the treatment of
Acanthamoeba keratitis.
Notes
-
We have no conflict of interest related to this study.
References
- 1. Parmar DN, Awwad ST, Petroll WM, Bowman RW, McCulley JP, Cavanagh HD. Tandem scanning confocal corneal microscopy in the diagnosis of suspected Acanthamoeba keratitis. Ophthalmology 2006;113:538-547.
- 2. Clarke DW, Neiderkorn JY. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol 2006;22:175-180.
- 3. Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome and risk factors. Br J Ophthalmol 2002;21:536-542.
- 4. Gagnon MR, Walter KA. A case of Acanthamoeba keratitis as a result of a cosmetic contact lens. Eye Contact Lens 2006;32:37-38.
- 5. Maghsood Att, Rezaian M, Rahimi F, Ghiasian SA, Famia SH. Contact lens associated Acanthamoeba keratitis in Iran. Iranian J Public Health 2005;34:40-47.
- 6. Roongruangchai K, Roongruangchai J, Kaewmanee S, Manoot D. Comparing lethal dose of povidone-iodine and Virkon to the Acanthamoeba cyst in vitro study. Siriraj Med J 2008;60:187-189.
- 7. Polat ZA, Tepe B, Vural A. In vitro effectiveness of Thymus sipyleus subsp. sipyleus var. sipyleus on Acanthamoeba castellanii and its cytotoxic potential on corneal cells. Parasitol Res 2007;101:1551-1555.
- 8. Goze I, Alim A, Dag S, Tepe B, Polat ZA. In vitro amoebicidal activity of Salvia staminea and Salvia caespitosa on Acanthamoeba castellanii and their cytotoxic potentials on corneal cells. J Ocul Pharmacol Ther 2009;25:293-298.
- 9. Chu DM, Miles H, Toney D, Ngyuen C, Marciano-Cabral F. Amebicidal activity of plant extracts from Southeast Asia on Acanthamoeba spp. Parasitol Res 1998;84:746-752.
- 10. Tepe B, Degerli S, Arslan S, Malatyali E, Sarikurkcu C. Determination of chemical profile, antioxidant, DNA damage protection and antiamoebic activities of Teucrium polium and Stachys iberica. Fitoterapia 2011;82:237-246.
- 11. Degerli S, Berk S, Malatyali E, Tepe B. Screening of the in vitro amoebicidal activities of Pastinaca armenea (Fisch. & C.A. Mey.) and Inula oculus-christi (L.) on Acanthamoeba castellanii cysts and tropho¬zoites. Parasitol Res 2011;110:565-570. doi:10.1007/s00436-011-2524-z
- 12. Malatyali E, Tepe B, Degerli S, Berk S, Akpulat HA. In vitro amoebicidal activity of four Peucedanum species on Acanthamoeba castellanii cysts and trophozoites. Parasitol Res 2011;110:167-174. doi:10.1007/s00436-011-2466-5
- 13. Derda M, Hadaś E, Thiem B. Plant extracts as natural amoebicidal agents. Parasitol Res 2009;104:705-708.
- 14. Ródio C, da Roch VD, Kowalski KP, Panatieri LF, von Poser G, Rott MB. In vitro evaluation of the amebicidal activity of Pterocaulon polystachyum (Asteraceae) against trophozoites of Acanthamoeba castellanii. Parasitol Res 2008;104:191-194.
- 15. Polat ZA, Vural A, Tepe B, Cetin A. In vitro amoebicidal activity of four Allium species on Acanthamoeba castellanii and their cytotoxic potentials on corneal cells. Parasitol Res 2007;101:397-402.
- 16. Polat ZA, Vural A, Ozan F, Tepe B, Özcelik S, Cetin A. In vitro evaluation of the amoebicidal activity of garlic (Allium sativum) extract on Acanthamoeba castellanii and its cytotoxic potential on corneal cells. J Ocul Pharmacol Ther 2008;24:8-14.
- 17. El-Sayed N, Ismail K, Ahmed S. In vitro amoebi¬cidal activity of ethanol extracts of Arachis hypogaea L., Curcuma longa L. and Pancratium maritimum L. on Acanthamoeba castellanii cysts. Parasitol Res 2011;110:1985-1992.
- 18. Díez J, Manjón JL, Martin F. Molecular phylogeny of the mycorrhizal desert truffles (Terfezia and Tirmania), host specificity and edaphic tolerance. Mycologia 2002;94:247-259.
- 19. Ermeli NB, Alsabri SG, Bensaber SM, Mohamed SB, Zetrini AA, Aburas KM, Fitouri SR, Jaeda MI, Mrema IA, Hermann A, Gbaj AM. Screening of analgesic and anti-inflammatory activities for two Libyan medicinal plants: Helianthemum lippii and Launaea residifolia. J Chem Pharm Res 2012;4:4201-4205.
- 20. Meckes M, Villareal ML, Tortoriello J, Berlin B, Berlin EA. A microbiological evaluation of medicinal plants used by the Maya people of Southern Mexico. Phytother Res 1995;9:244-250.
- 21. Meckes M, Torres J, Calzada F, Rivera J, Camorlinga M, Lemus H, Rodríguez G. Antibacterial properties of Helianthemum glomeratum, a plant used in Maya traditional medicine to treat diarrhea. Phytother Res 1997;11:128-131.
- 22. Calzada F, Alanís AD, Meckes M, Tapia-Contreras A, Cedillo-Rivera R. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to some medicinal plants used by the people of Southern Mexico. Phytother Res 1998;12:70-72.
- 23. Calzada F, Lopéz R, Meckes M, Cedillo-Rivera R. Flavonoids of the aerial parts of Helianthemum glomeratum. Int J Pharmacog 1995;33:351-352.
- 24. Meckes M, Calzada F, Tapia-Contreras A, Cedillo-Rivera R. Antiprotozoal properties of Helianthemum glomeratum. Phytother Res 1999;13:102-105.
- 25. Perumalla S, Nayeem N. Determination of total phenolic acids, condensed tannins and flavonoids in the leaves of Caesalpinia pulcherrima Linn. Int J Phytother Res 2012;2:16-19.
- 26. Kosalec I, Bakmaz M, Pepeljnjak S, Vladimir-Knezevic S. Quantitaive analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm 2004;54:65-71.
- 27. Vanburen JP, Robinson WB. Formation of complexes between protein and tannic acid. J Agricul Food Chem 1969;17:772-777.
- 28. Init I, Lau YL, Arin Fadzlun A, Foead AI, Neilson RS, Nissapatorn V. Detection of free living amoebae, Acanthamoeba and Naegleria, in swimming pools, Malaysia. Trop Biomed 2010;27:566-577.
- 29. Perrine D, Chenu JP, Georges P, Lancelot JC, Saturnino C, Robba M. Amoebicidal efficiencies of various diamidines against two strains of Acanthamoeba polyphaga. Antimicrob Agents Chemother 1995;39:339-342.
- 30. Ledee DR, Hay J, Byers TJ, Seal DV, Kirkness CM. Acanthamoeba griffini molecular characterization of a new corneal pathogen. Invest Ophthalmol Vis Sci 1996;37:544-550.
- 31. Shoaib HM, Muazzam AG, Mir A, Jung S, Matin A. Evaluation of inhibitory potential of some selective methanolic plants extracts on biological characteristics of Acanthamoeba castellanii using human corneal epithelial cells in vitro. Parasitol Res 2013;112:1179-1188. doi:10.1007/s00436-012-3249-3
- 32. Shinwari ZK. Medicinal plants research in Pakistan. J Med Plan Res 2010;4:161-176.
- 33. Rubio-Moraga A, Argandoña J, Mota B, Pérez J, Verde A, Fajardo J, Gómez-Navarro J, Castillo-López R, Ahrazem O, Gómez-Gómez L. Screening for polyphenols, antioxidant and antimicrobial activitiesof extracts from eleven Helianthemum taxa (Cistaceae) used in folk medicine in south-eastern Spain. J Ethnopharmacol 2013;148:287-296.
- 34. Okuda T, Yoshida T, Hatano T. Ellagitannins as active constituents of medicinal plants. Planta Med 1989;55:117-122.
- 35. Stammler G, Volm M. Green tea catechins (EGCG and EGC) have modulating effects on the activity of doxorubicin in drug-resistant cell lines. Anticancer Drugs 1997;8:265-268.
Fig. 1Standard curves of gallic acid using Folin-Ciocalteu reagent (A), quercetin (B), and gallic acid using FeCl3 (C) for phytochemical screening of ethyl acetate extract of Helianthemum lippii (L.) (D).

Fig. 2Light microscopy of non-viable cysts (A) and viable cysts (B) of Acanthamoeba castellanii treated by Helianthemum lippii (L.) extracts.

Fig. 3Comparison between ethyl acetate and methanol extracts as regard to the percentage of non-viable cysts at days 1, 2, and 3.

Table 1Comparison between ethyl acetate and methanol extracts versus negative (non-treated parasite) and positive (chlorohexidine) controls with regard to the percentage of non-viable cysts at days 1, 2, and 3
Table 1
|
Ethyl acetate extract
|
Methanol extract
|
PAa
|
Negative control
|
Positive control
|
Ethyl acetate extract
|
Methanol extract
|
|
Mean±SD |
Mean±SD |
Negative control P-valuec
|
Positive control P-valuec
|
Negative control P-valuec
|
Positive control P-valuec
|
|
Day 1 |
28.0±9.3 |
19.5± 11.3 |
0.011 |
6.4±0.28 |
7.4 ±1.13 |
0.0001 |
0.0001 |
0.0001 |
0.0001 |
|
Day 2 |
37.2±13.6 |
25.0±12.1 |
0.004 |
14.3±0.21 |
30.2±2.6 |
0.0001 |
0.029 |
0.001 |
0.062 |
|
Day 3 |
47.5±22.9 |
24.0±18.4 |
0.001 |
22.3±0.21 |
53.1 ±4.1 |
0.0001 |
0.278 |
0.677 |
0.0001 |
|
P-valueb
|
0.001 |
0.27 |
|
|
|
|
|
|
|
Table 2.Comparison between different concentrations of ethyl acetate and methanol extracts with regard to the percentage of non-viable cysts at days 1, 2, and 3
Table 2.
|
|
Ethyl acetate extract
|
|
Methanol extract
|
|
|
Mean |
SD |
P-valuea
|
|
Mean |
SD |
P-valuea
|
|
Day 1 |
C1 |
24.0 |
7.0 |
0.3 |
C1 |
33.6 |
5.5 |
0.0001 |
|
C2 |
22.0 |
5.1 |
|
C2 |
22.3 |
7.5 |
|
|
C3 |
36.3 |
12.7 |
|
C3 |
21.3 |
7.0 |
|
|
C4 |
20.6 |
3.5 |
|
C4 |
32.3 |
1.1 |
|
|
C5 |
32.0 |
4.5 |
|
C5 |
10.0 |
6.0 |
|
|
C6 |
29.0 |
11.3 |
|
C6 |
8.3 |
2.0 |
|
|
C7 |
32.0 |
12.1 |
|
C7 |
8.6 |
5.6 |
|
|
Day 2 |
C1 |
50.3 |
16.7 |
0.01 |
C1 |
11.3 |
1.5 |
0.0001 |
|
C2 |
43.0 |
12.1 |
|
C2 |
37.6 |
6.3 |
|
|
C3 |
53.0 |
8.8 |
|
C3 |
34.0 |
7.0 |
|
|
C4 |
25.0 |
8.7 |
|
C4 |
30.0 |
1.0 |
|
|
C5 |
31.3 |
3.2 |
|
C5 |
36.6 |
3.7 |
|
|
C6 |
29.3 |
6.5 |
|
C6 |
12.0 |
1.0 |
|
|
C7 |
28.3 |
6.4 |
|
C7 |
13.0 |
3.6 |
|
|
Day 3 |
C1 |
75.0 |
21.9 |
0.002 |
C1 |
6.0 |
1.0 |
0.003 |
|
C2 |
51.0 |
1.7 |
|
C2 |
45.3 |
14.0 |
|
|
C3 |
73.0 |
27.7 |
|
C3 |
38.6 |
14.1 |
|
|
C4 |
38.3 |
7.0 |
|
C4 |
14.6 |
0.5 |
|
|
C5 |
17.0 |
2.6 |
|
C5 |
39.6 |
22.5 |
|
|
C6 |
41.6 |
7.2 |
|
C6 |
10.6 |
3.2 |
|
|
C7 |
36.6 |
2.3 |
|
C7 |
13.0 |
6.5 |
|