Abstract
In order to explore tick proteins as potential targets for further developing vaccine against ticks, the total proteins of unfed female Dermacentor silvarum were screened with anti-D. silvarum serum produced from rabbits. The results of western blot showed that 3 antigenic proteins of about 100, 68, and 52 kDa were detected by polyclonal antibodies, which means that they probably have immunogenicity. Then, unfed female tick proteins were separated by 12% SDS-PAGE, and target proteins (100, 68, and 52 kDa) were cut and analyzed by LC-MS/MS, respectively. The comparative results of peptide sequences showed that they might be vitellogenin (Vg), heat shock protein 60 (Hsp60), and fructose-1, 6-bisphosphate aldolase (FBA), respectively. These data will lay the foundation for the further validation of antigenic proteins to prevent infestation and diseases transmitted by D. silvarum.
-
Key words: Dermacentor silvarum, tick, antigenic protein, LC-MS/MS
Ticks are an obligate bloodsucking ectoparastism animal, which spread a variety of diseases and bring great harm to human health and development of the livestock [
1]. Now tick control is principally dependent on the application of acaricides. However, it can develop resistance to acaricides for ticks and raises environmental contamination [
2]. Therefore, vaccination is considered as one of the most effective ways for tick control [
3]. The key step is to screen antigenic proteins for further developing vaccine against ticks. Willadsen et al. [
4] isolated a midgut antigen Bm86 from
Rhipicephalus (
Boophilus)
microplus, and immune experiment results showed that Bm86 can effectively induce the host to produce protective immune responses. Galay et al. [
5] purified recombinant ferritins (rHlFER1 and rHlFER2) from
Haemaphysalis longicornis and immunized rabbits with them, respectively. The results of tick infestation challenge showed that rHlFER2 could significantly reduce the body weight of ticks infested from the rHlFER2-immunized rabbit compared to those from the control rabbits. Through mass spectrometry, Kim et al. [
6] and Hu et al. [
7] identified calreticulin and paramyosin (Pmy) from
H. longicornis, respectively. These scholars have been trying to screen more effective antigenic proteins in the preparation of anti-tick vaccines.
Dermacentor silvarum is one of the principal vectors of Russian spring encephalitis, Babesia equi, Babesia caballi, and rickettsiosis and is widely distributed in northern China, Russia, and Mongolia [
8]. However, only subolesin (4D8) was cloned from
D. silvarum, and other antigens were not reported in it [
9]. Here, new antigenic proteins were screened and identified from unfed female
D. silvarum by LC-MS/MS technology. This provides the foundation for the further development of anti-tick vaccines.
Adult
D. silvarum were collected from infested sheep at the Xiaowutai National Natural Conservation Area, Hebei Province, China and reared as described previously [
10]. Polyclonal antibodies against
D. silvarum were generated in adult male New Zealand white rabbits purchased from the Hebei Laboratory Animal Center (Shijiazhuang, China). Firstly, 20 unfed female ticks were fast frozen in liquid nitrogen and ground to powder, which were transferred into the tube containing 1 ml 0.1 M PBS (pH 7.2). Then, samples were centrifuged at 13,000 rpm for 10 min at 4˚C, and 360 μg supernatant extracts emulsified in an equal volume of Freund’s complete adjuvant were initially injected into the rabbits. Two additional injections were given every 2 weeks with 360 μg proteins emulsified with an equal volume of Freund’s incomplete adjuvant. One week after the third injection, blood was collected from rabbits, and antibodies in serum were purified by caprylic acid-ammonium sulfate method and stored at -70˚C for later analysis.
The screening of antigenic proteins in
D. silvarum was performed by western blot. A total of 5 unfed female ticks were frozen in liquid nitrogen and ground to powder, which were transferred to a tube containing 1 ml 0.1 M PBS. Then, the samples were centrifuged at 13,000 rpm for 10 min at 4˚C, and supernatant extracts were stored. About 30 μg protein per lane, including prestained protein marker (Fermentas, Ontario, Canada), were separated by 12% SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. Then, the membrane was incubated with polyclonal antibodies (1:2,000) and rabbit negative serum overnight at room temperature, respectively. After incubation, they were washed with PBS-Tween-20 (PBST) and incubated with peroxidase-conjugated affinipure goat anti-rabbit IgG (1:2,000) for 1.5 hr (Proteintech, Chicago, Illinois, USA). Antigenic proteins were detected using supersignal
® west dura extended duration substrate (Thermo Scientific, Rockford, Illinois, USA). To identify the antigenic proteins, 30 μg protein from unfed female ticks was electrophoresed on a 12% SDS-PAGE gel and stained with Commassie blue. Based on the molecular weight marker, the target protein bands were cut and placed into 2 ml tubes, respectively, for LC-MS/MS analysis. The method was the same as described previously [
7].
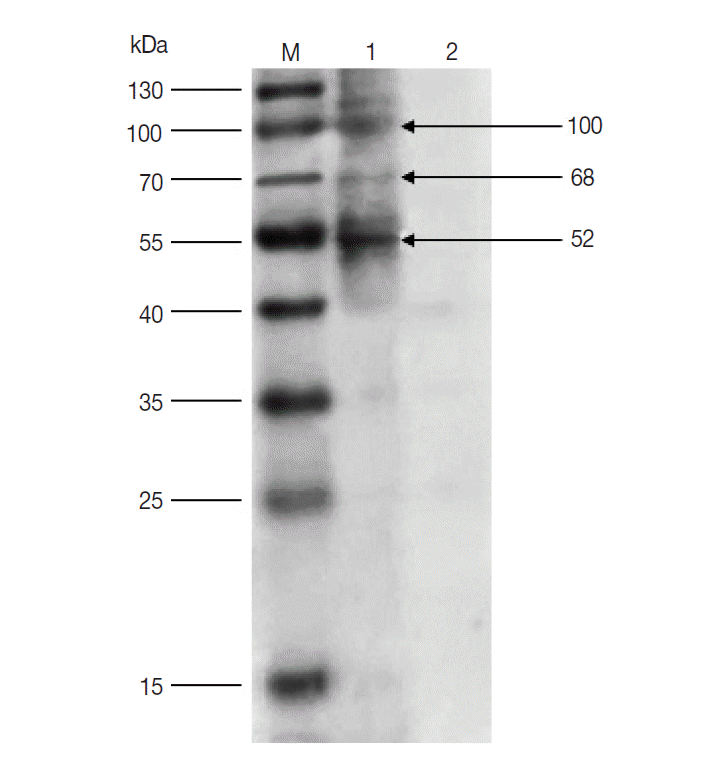
The results of western blot showed that 100, 68, and 52 kDa protein bands were recognized by rabbit anti-
D. silvarum serum, while the rabbit negative serum did not react with the female tick proteins (
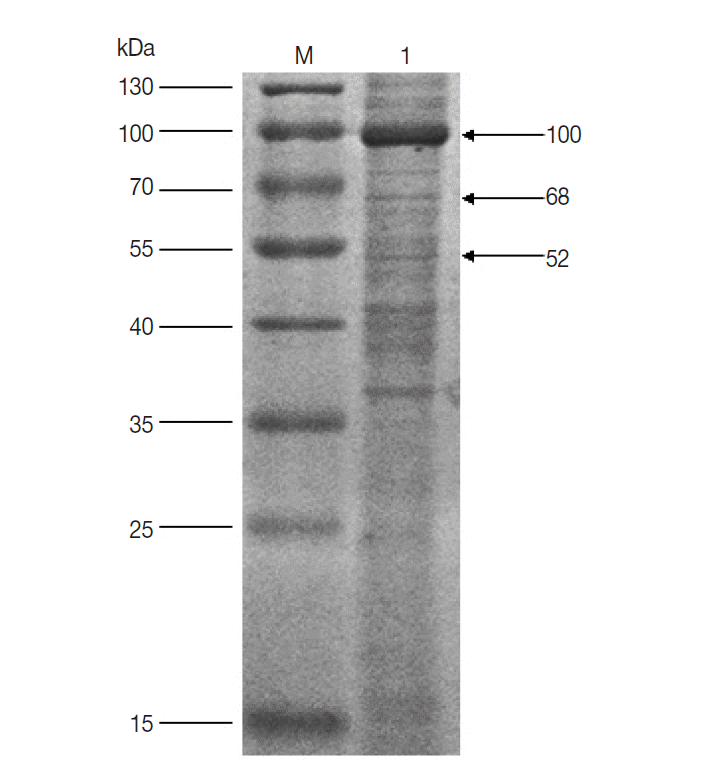
Fig. 1). It suggested that the 100, 68, and 52 kDa proteins had potential immunogenic properties that might be candidate antigens for developing a vaccine. Three target protein bands were excised, respectively, from Commassie-stained SDS-PAGE gel (
Fig. 2) and analyzed by LC-MS/MS. The data were searched in the National Center for Biotechnology Information (NCBI) database, and many amino acid sequences of peptides derived from target proteins matched the amino acid sequence of ticks (
Table 1). It means that the 100, 68 and 52 kDa protein may be the vitellogenin (Vg), heat shock protein 60 (Hsp60), and fructose-1, 6-bisphosphate aldolase (FBA), respectively.
The screening of antigenic proteins is necessary for developing an effective vaccine against the tick infection. By western blot analysis, 5 midgut membrane antigens with molecular weight 95, 85, 66, 49, and 42 kDa had been identified in
Hyalomma anatolicum anatolicum [
11]. Through mass spectrometry, calreticulin and Pmy were identified in
H. longicornis [
6,
7]. By the similar method, 3 proteins (100, 68, and 52 kDa) from
D. silvarum were demonstrated to have immunogenicity, and the results of LC-MS/MS showed that they might be Vg, Hsp60, and FBA (
Fig. 1;
Table 1).
Firstly, Vg is a phospholipoglycoprotein, which constitutes the precursor of vitellin (Vn) in all oviparous organisms. In vitellogenic female insects, Vg is generally synthesized by the fat body, released into the hemolymph and transported to the ovary, where it is proteolytically cleaved to form the Vn, providing the energy for developing embryos [
12,
13]. Additionally, Vg has been demonstrated to have capability of killing bacteria via interaction with lipopolysaccharide and lipoteichoic acid [
14]. Moreover, Tellam et al. [
15] purified Vn from B.
microplus and vaccinated sheep with them. The results of the vaccination trial showed that the mean percentage engorgement of ticks fed on the immunized sheep was reduced from 72% to 42%, and the weights and oviposition of engorged female ticks were significantly reduced compared with the control group. The mechanism would be that host antibodies against Vn/Vg flowed from the tick midgut lumen to the hemolymph, where they bound to Vg, thus blocking Vg uptake by oocytes in the ovary and affecting the development of ticks [
16,
17]. In our results, for the first time,
D. silvarum Vg was recognized by sera from rabbits immunized with tick lysates. As a vaccine candidate, antigenic significance of this protein was verified by many works.
The alternate name of Hsp60 is called chaperonin 60, which is a mitochondrial chaperonin in eukaryotic cells, and known as phage growth E large (GroEL) in bacteria [
18]. In addition to its role as a heat shock protein, Hsp60 functions as a chaperonin to bind partially folded proteins and mediates their conformational changes [
19]. Additionally, Hsp60 act directly on antigen-presenting cells (APC) to link innate and adaptive immune responses [
20]. Ben et al. [
21] found that immunization of mice with
Strongyloides ratti Hsp60 (srHSP60) led to reduced numbers of migrating larvae, parasitic adults and larval output of
S. ratti, and protected mice against nematode challenge infection. Moreover, immunization of female BALB/c mice with recombinant protein NP-M1-HSP60 induced immune responses, and protected mice from lethal influenza H7N9 virus challenge and significantly inhibited viral replication [
22]. In our previous studies [
7], 66 kDa proteins from
H. longicornis had immunogenic properties, which might be Hsp60 verified later by LC-MS/MS. We also found similar results in the experiments of screening antigenic proteins from
D. silvarum. Based on current knowledge, Hsp60 will be examined as a possible target for the development of novel anti-
D. silvarum vaccine.
As we all know, FBA is one of the most important enzymes in the glycolytic pathway and catalyzes the reversible cleavage of fructose-1,6-bisphosphate to dihydroxyacetone phosphate and glyceraldehyde 3-phosphate [
23]. FBA was also identified as an immunogenic protein in
Streptococcus pneumoniae. Ling et al. [
24] separated proteins from
S. pneumoniae by 2D gel electrophoresis, and immunogenic protein FBA from the cell wall fraction were identified by MS. Moreover, recombinant FBA (rFBA) was immunogenic in mice and able to provide partial protection against virulent strain of
S. pneumoniae. Thus, their conservation, surface-localization, and immunogenicity may make FBA potentially useful vaccine candidates for a range of pathogens [
25]. By the similar method, we also screened the FBA from
D. silvarum. In conclusion, 3 antigenic proteins (100, 68, and 52 kDa) were identified in our study. For the detection of protective immunogenicity, immunization of experimental animals with them will be done in our next work. This will provide a basis for developing a candidate vaccine to prevent infestation and diseases transmitted by
D. silvarum.
Notes
-
We have no conflict of interest related to this work.
This work was supported by National Natural Science Foundation of China (no. 31402022), China Postdoctoral Science Foundation (no. 2014M551042), the Specialized Research Fund for the Doctoral Program of Higher Education of China (no. 20131303120008) and Natural Science Foundation of Hebei Province (no. C2015205126).
Fig. 1.Western blot of unfed female tick proteins from D. silvarum. M, the prestained protein marker; Lane 1, unfed female tick proteins incubated by polyclonal antibodies from rabbits immunized with tick lysates; Lane 2, unfed female tick proteins incubated by rabbit negative serum.
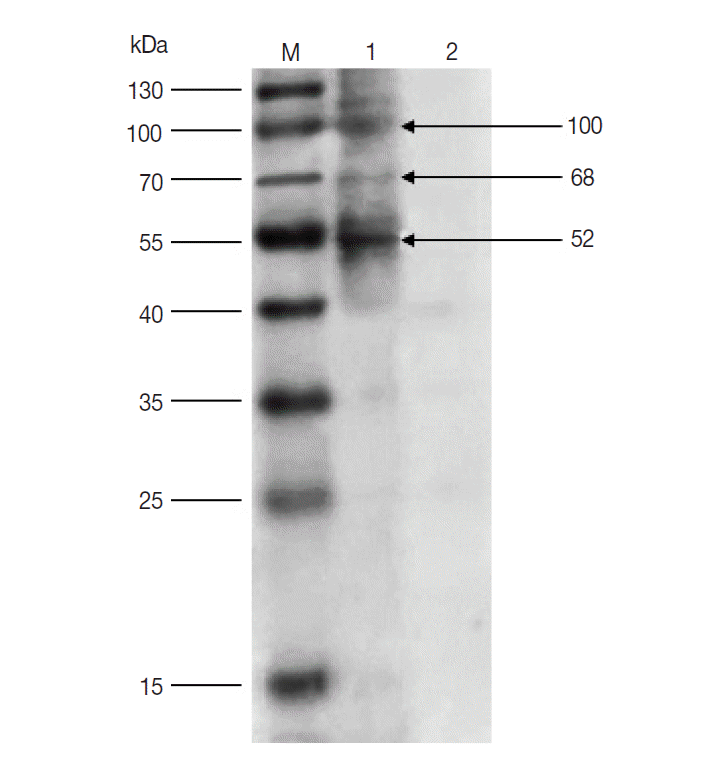
Fig. 2.SDS-PAGE analysis of unfed female tick proteins of D. silvarum. M, the molecular weight marker; Lane 1, unfed female tick proteins of D. silvarum. The target proteins (100, 68, and 52 kDa) shown by the arrow were cut, respectively, for LC-MS/MS analysis.
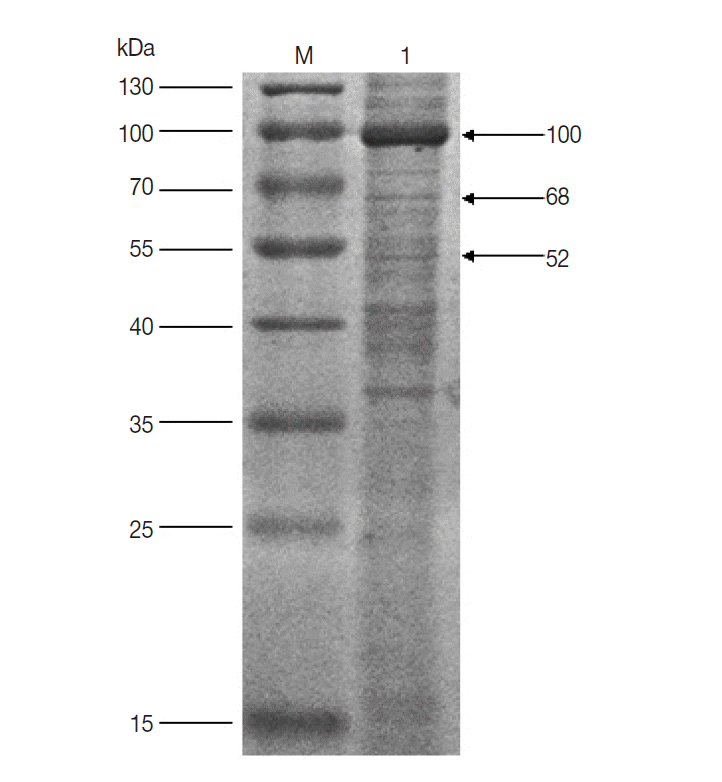
Table 1.Peptides of the target proteins identified by LC-MS/MS from unfed female D. silvarum
Table 1.
|
Spot no. |
Reference
|
P (pro)
|
Score
|
|
scan (s) |
Peptide |
MH+ |
DeltaM |
z |
Typ |
P (pep) |
XC |
|
100 kDa |
gi|86371135|gb|ABC94727.1| vitellogenin [Dermacentor variabilis] |
|
|
|
|
|
5.53E-07 |
50.18 |
|
4777 |
R.LVGPQPGSTK.N |
983.55202 |
0.3519 |
2 |
CID |
7.17E-06 |
2.05 |
|
7717 |
R.YVLPLWETNPR.F |
1387.73686 |
0.50244 |
2 |
CID |
4.96E-06 |
3.59 |
|
10433 |
R.HIGLEVLSDPSDQVVAFVISAFR.A |
2499.3242 |
1.81965 |
2 |
CID |
5.53E-07 |
2.72 |
|
68 kDa |
gi|241998158|ref|XP_002433722.1| chaperonin subunit (groEL), putative [Ixodes scapularis] |
|
|
0.39943 |
|
CID |
1.35E-06 |
10.16 |
|
4934 |
K.VGGSSEVEVNEK.K |
1233.59574 |
|
|
|
4.93E-05 |
3.79 |
|
4944 |
K.VGGSSEVEVNEK.K |
1233.59574 |
-0.05431 |
2 |
CID |
3.48E-06 |
3.74 |
|
52 kDa |
gi|241690479|ref|XP_002411768.1| fructose 1,6-bisphosphate aldolase, [Ixodes scapularis] |
|
|
|
|
|
1.30E-09 |
40.21 |
|
5004 |
K.KYTPADVAR.A |
1020.54727 |
1.42043 |
2 |
CID |
5.39E-03 |
2.75 |
|
5505 |
R.LQGIGVENTEENRR.Q |
1614.81942 |
-0.39579 |
2 |
CID |
2.60E-03 |
2.64 |
|
5607 |
R.LQGIGVENTEENR.R |
1458.71831 |
0.40454 |
2 |
CID |
2.44E-08 |
4.19 |
References
- 1. Ahmed J, Alp H, Aksin M, Seitzer U. Current status of ticks in Asia. Parasitol Res 2007;101:S159-S162.
- 2. Davey RB, George JE, Miller RJ. Comparison of the reproductive biology between acaricide-resistant and acaricide-susceptible Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet Parasitol 2006;139:211-220.
- 3. Nuttall PA, Trimnell AR, Kazimirova M, Labuda M. Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite Immunol 2006;28:155-163.
- 4. Willadsen P, Riding GA, Mckenna RV, Kemp DH, Tellam RL, Nielsen JN, Lahnstein J, Cobon GS, Gough JM. Immunologic control of a parasitic arthropod: identification of a protective antigen from Boophilus microplus. J Immunol 1989;143:1346-1351.
- 5. Galay RL, Miyata T, Umemiya-Shirafuji R, Maeda H, Kusakisako K, Tsuji N, Mochizuki M, Fujisaki K, Tanaka T. Evaluation and comparison of the potential of two ferritins as anti-tick vaccines against Haemaphysalis longicornis. Parasit Vectors 2014;7:482.
- 6. Kim YH, Slam MS, You MJ. Proteomic screening of antigenic proteins from the hard tick, Haemaphysalis longicornis (Acari: Ixodidae). Korean J Parasitol 2015;53:85-93.
- 7. Hu Y, Zhang J, Yang S, Wang H, Zeng H, Zhang T, Liu J. Screening and molecular cloning of a protective antigen from the midgut of Haemaphysalis longicornis. Korean J Parasitol 2013;51:327-334.
- 8. Yu Z, Zheng H, Chen Z, Zheng B, Ma H, Liu J. The life cycle and biological characteristics of Dermacentor silvarum Olenev (Acari: Ixodidae) under field conditions. Vet Parasitol 2010;168:323-328.
- 9. Hu Y, Zeng H, Zhang J, Wang D, Li D, Zhang T, Yang S, Liu J. Gene cloning, expression and immunogenicity of the protective antigen subolesin in Dermacentor silvarum. Korean J Parasitol 2014;52:93-97.
- 10. Liu J, Liu Z, Zhang Y, Yang X, Gao Z. Biology of Dermacentor silvarum (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 2005;36:131-138.
- 11. Madani R, Golchinfar F, Dadmehr A, Abdigoudarzi M. Preparation of antigens from midgut of Hyalomma anatolicum anatolicum and determining their immunoprotective effects. Arch Razi Institute 2009;63:47-52.
- 12. Raikhel AS, Dhadialla TS. Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol 1992;37:217-251.
- 13. Sappington TW, Raikhel AS. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem Mol Biol 1998;28:277-300.
- 14. Li Z, Zhang S, Zhang J, Liu M, Liu Z. Vitellogenin is a cidal factor capable of killing bacteria via interaction with lipopolysaccharide and lipoteichoic acid. Mol Immunol 2009;46:3232-3239.
- 15. Tellam RL, Kemp D, Riding G, Briscoe S, Smith D, Sharp P, Irving D, Willadsen P. Reduced oviposition of Boophilus microplus feeding on sheep vaccinated with vitellin. Vet Parasitol 2002;103:141-156.
- 16. Chinzei Y, Minoura H. Reduced oviposition in Ornithodoros moubata (Acari: Argasidae) fed on tick-sensitized and vitellin-immunized rabbits. J Med Entomol 1988;25:26-31.
- 17. Gudderra NP, Sonenshine DE, Apperson CS, Roe RM. Haemolymph proteins in ticks. J Insect Physiol 2002;48:269-278.
- 18. Georgopoulos C, Welch W. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol 1993;9:601-634.
- 19. Martin J, Horwich AL, Hartl FU. Prevention of protein denaturation under heat stress by the chaperonin Hsp60. Science 1992;258:995-998.
- 20. Moré SH, Breloer M, von Bonin A. Eukaryotic heat shock proteins as molecular links in innate and adaptive immune responses: Hsp60-mediated activation of cytotoxic T cells. Int Immunol 2001;13:1121-1127.
- 21. Ben Nouir N, Piédavent M, Osterloh A, Breloer M. Passive immunization with a monoclonal IgM antibody specific for Strongyloides ratti HSP60 protects mice against challenge infection. Vaccine 2012;30:4971-4976.
- 22. Yang P, Wang W, Gu H, Li Z, Zhang K, Wang Z, Li R, Duan Y, Zhang S, Wang X. Protection against influenza H7N9 virus challenge with a recombinant NP-M1-HSP60 protein vaccine construct in BALB/c mice. Antiviral Res 2014;111:1-7.
- 23. Zhang L, Guo Z, Huang J, Liu M, Wang Y, Ji C. Expression, purification, crystallization and preliminary X-ray crystallographic analysis of fructose-1,6-bisphosphate aldolase from Escherichia coli. Acta Crystallogr F Struct Biol Commun 2014;70:1376-1379.
- 24. Ling E, Feldman G, Portnoi M, Dagan R, Overweg K, Mulholland F, Chalifa-Caspi V, Wells J, Mizrachi-Nebenzahl Y. Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse. Clin Exp Immunol 2004;138:290-298.
- 25. Shams F, Oldfield NJ, Wooldridge KG, Turner DP. Fructose-1,6-bisphosphate aldolase (FBA)-a conserved glycolytic enzyme with virulence functions in bacteria: 'ill met by moonlight'. Biochem Soc Trans 2014;42:1792-1795.