Abstract
A scanning electron microscopic study was performed on the surface ultrastructure of Pygidiopsis summa (Digenea: Heterophyidae) adults. Metacercariae were collected from gills and muscles of mullets (Mugil cephalus) caught in a known endemic area, and adult flukes were harvested from dogs after 8 weeks of experimental infection. The worm was calabash form with its posterior part broader than the anterior part. Tegumental spines were densely distributed over the body surface, except on the suckers and genital apparatus, and around the excretory pore. Well differentiated spines were observed on the anterior half of the body, with 14-16 tips ventrally, and 19-20 tips dorsally. On the oral sucker, three pairs of type I sensory papillae (uni-ciliated knob-like swellings) and one pair of type II sensory papillae (aciliated round-swellings) were observed on the anterior and posterior parts of the lip, respectively. On the lip of the ventral sucker, one pair of type II sensory papillae was distributed only on its posterior part. Sperms were seen emerging from or entering into the genital apparatus. The results showed that the surface ultrastructure of P. summa was unique among the heterophyid trematodes, especially in digitation of tegumental spines and in distribution of sensory papillae on oral and ventral suckers.
-
Key words: Pygidiopsis summa, Heterophyidae, microscopy (electron, scanning), body surface area
INTRODUCTION
Pygidiopsis summa (Digenea: Heterophyidae) was originally described in Japan from dogs experimentally fed mullets infected with the metacercariae (
Onji and Nishio, 1916). Human infections were first found by detection of eggs in the feces in Japan (
Takahashi, 1929), and adult flukes from humans were identified later (
Yokogawa et al., 1965). This parasite is now known to distribute in the Republic of Korea (
Chun, 1963;
Seo et al., 1981a,
1981b;
Chai and Lee, 1990;
Sohn et al., 1994), and at least 31 worm-proven human cases have been reported (
Seo et al., 1981a;
Chai et al., 1997,
1998a). The adult flukes are morphologically characterized by their small concave body, median location of the ventral sucker, unique morphology of the ventrogenital apparatus, and side-by-side location of two testes (
Chai et al., 1986).
Surface ultrastructures of helminth parasites, as revealed by scanning electron microscopy (SEM), are helpful for not only taxonomic studies but also for immunological and drug efficacy studies. In heterophyid trematodes, SEM studies have been performed in
Cryptocotyle lingua (
Koie, 1977),
Heterophyes aequalis (
Taraschewski, 1984),
H. nocens (
Chai et al., 1992),
Heterophyopsis continua (
Hong et al., 1991),
Metagonimus yokogawai (
Lee et al., 1984),
M. miyatai (
Chai et al., 1998b), and
M. takahashii (
Chai et al., 2000). However, no information is available for
P. summa. Thus, the present study was performed to observe the surface ultrastructure of
P. summa adults and to compare the results with those reported for other heterophyid trematodes.
MATERIALS AND METHODS
Metacercariae of P. summa were isolated from gills and muscles of the mullet (Mugil cephalus) caught at an estuary in Okku-gun, Jeonrabuk-do (Province), by artificial digestion technique. Adult flukes were harvested from the small intestine of experimentally infected dogs at week 8 post-infection (PI). The adult flukes were washed with 0.2M cacodylate buffer (pH 7.3) and fixed in 2.5% glutaraldehyde. They were dehydrated, dried, and mounted on aluminum stubs, followed by coat with gold. The specimens were observed with a scanning electron microscope (ISI DS-130C, Korea) at an accelerating voltage of 10 kV.
RESULTS
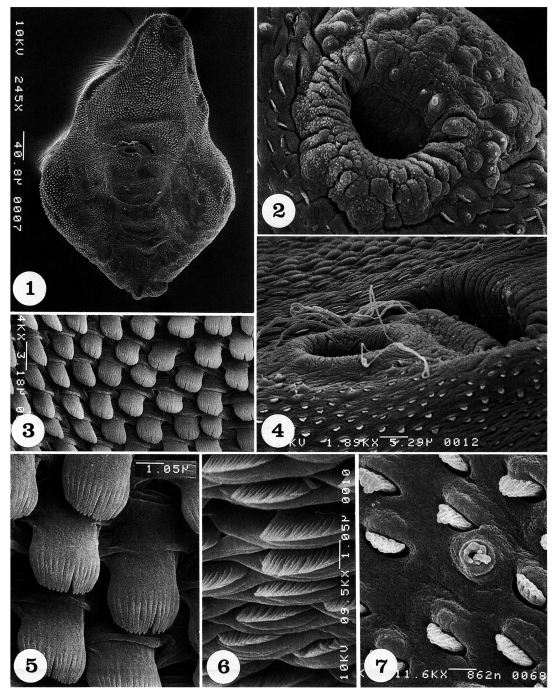
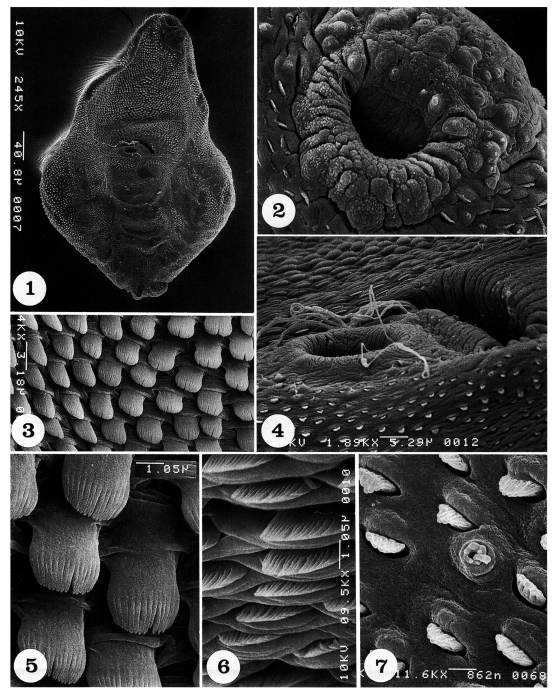
Adult flukes are ventrally concave, calabash or pyriform in shape, and equipped with oral, ventral suckers, and a genital apparatus (
Fig. 1). The whole body surface is covered with flat cytoplasmic processes, numerous tegumental spines, and sensory papillae, as seen from ventral (
Figs. 1-7) and dorsal views (
Figs. 8-14).
The oral sucker is small and located near the anterior end (
Fig. 2). It has one pair of small type I sensory papillae (ciliated knob-like swellings) and two pairs of large type I sensory papillae on the anterior part of the lip, and one pair of type II papillae (aciliated round-swellings) on its posterior lip (
Fig. 2). On the tegument between oral and ventral suckers, spines are densely distributed; they are long, painting brush-shaped, and having 14-16 digits (
Figs. 3 & 5). The ventral sucker is small, submedian, protruded ventrally, and equipped with one pair of type II sensory papillae on its postero-lateral margins (
Fig. 4). Just anterior to the ventral sucker, the genital apparatus is seen as a transversely long furrow, and sperms are seen escaping from or entering into the genital pore (
Fig. 4). On the tegument anterior to the genital apparatus, spines are short, broom brush-shaped, and having 14-16 digits at the tip (
Fig. 6). The spines on the postero-ventral body surface are sparsely distributed, and they become spade-shape with smaller numbers of digitations at the tip (
Fig. 7).
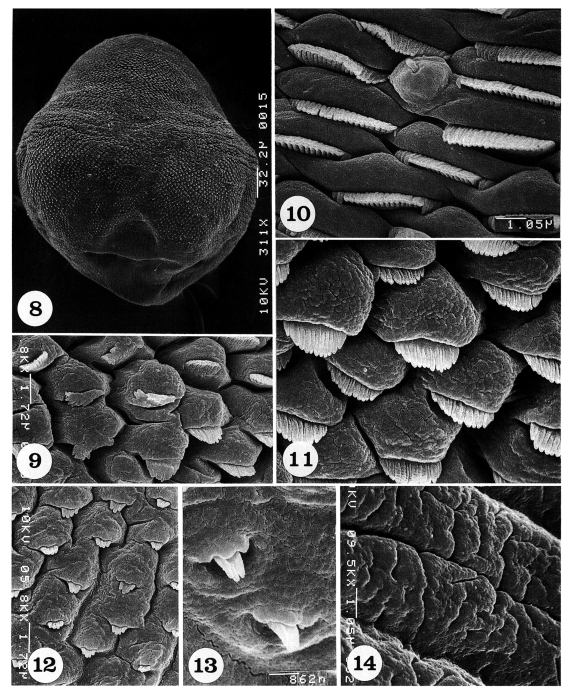
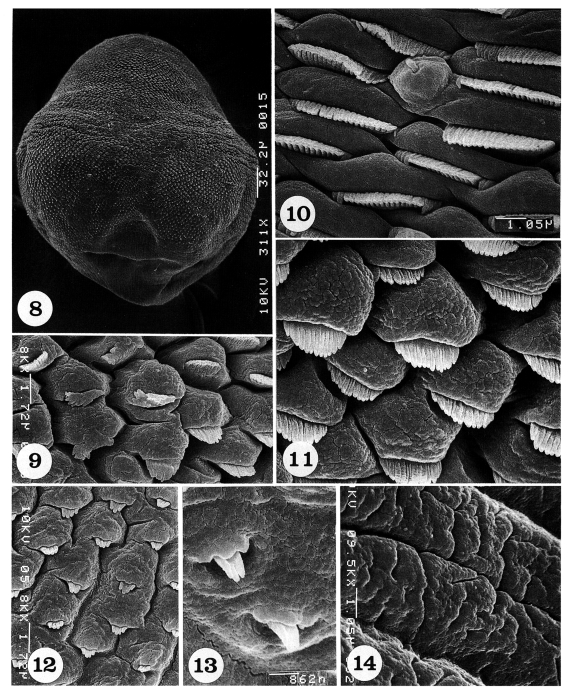
The dorsal body surface is covered densely with tegumental spines and sensory papillae, except around the posterior end of the body and excretory pore (
Fig. 8).
The antero-lateral body surface is covered with protruded cytoplasmic processes, each with a 15-17 pointed tegumental spine (
Fig. 9). On the mid-lateral surface, tegumental spines become more densely crowded and wider in shape with 19-21 pointed tips; uni-ciliated sensory papillae are at times observed (
Fig. 10). In some areas of the mid-lateral surface, the cytoplasmic processes are protruded, and each process bears a spade-shaped tegumental spine with 12-14 digits (
Fig. 11). On the mid-posterior body surface, the tegumental spines are sparse and claw-shaped with 4-5 digits (
Fig. 12). On the postero-lateral body surface, spines are also sparsely distributed and claw-shaped with 3-4 digits (
Fig. 13). The tegument near the posterior end of the body is severely wrinkled with deep furrows and devoid of spines (
Fig. 14).
DISCUSSION
The surface ultrastructures of
P. summa were generally similar to that of other heterophyid flukes;
M. yokogawai (
Lee et al., 1984),
M. miyatai (
Chai et al., 1998b),
M. takahashii (
Chai et al., 2000),
H. continua (
Hong et al., 1991), and
H. nocens (
Chai et al., 1992). However, significant differences were recognized in the morphology and distribution of tegumental spines and sensory papillae.
In
P. summa, well-differentiated spines had 14-16 pointed tips ventrally, and 19-21 pointed tips dorsally. In
Metagonimus spp., digitation of the spines was less; 7-9 (ventrally) and 9-11 tips (dorsally) in
M. yokogawai (
Lee et al., 1984), 9-11 (ventrally) and 10-12 tips (dorsally) in
M. miyatai (
Chai et al., 1998b), and 10-12 (ventrally) and 10-13 tips (dorsally) in
M. takahashii (
Chai et al., 2000). In
H. continua, tegumental spines on the ventral or dorsal surface are with 15-17 pointed tips (
Hong et al., 1991), but less than that in
P. summa. In
H. nocens, the spines on the ventral surface are 12-17 pointed, and those on the dorsal surface are 15-20 pointed (
Chai et al., 1992). Therefore, digitation of the spines of
P. summa resembles that of
H. nocens.
The shape of tegumental spines is a characteristic feature in the surface ultrastructure of heterophyid flukes. In
P. summa, the spines were, in most cases, broom brush- or painting brush-shaped, and in some areas, they were spade-shaped. Near the posterior extremity, the spines were mostly bear-claw-shaped. In
Metagonimus spp. and
H. nocens, the spines are normally spade- or round brush-shaped (
Lee et al., 1984;
Chai et al., 1998b,
2000). Broom brush-shaped spines, similar to those observed in this study, have also been observed in
H. continua (
Hong et al., 1991). However, the presence of painting brush-shaped or bear-claw-shaped spines in
P. summa seemed to be a unique feature among different species of heterophyid flukes.
Two types of sensory papillae, type I and type II, were observed in
P. summa. These papillae have been found as common features among heterophyid flukes. Type III sensory papillae (round swelling of cytoplasmic ridges), which were observed on the oral sucker of
M. yokogawai (
Lee et al., 1984), were absent in
M. miyatai (
Chai et al., 1998b),
M. takahashii (
Chai et al., 2000) and
P. summa (this study).
It is worth to mention here that, light microscopically, minute spines are present on the gonotyl (5 on the right side and 9 on the left side) of the genital apparatus in 7-day adults of
P. summa (
Chai et al., 1986). We tried to observe the detailed morphology of the spines by scanning electron microscopy, but failed to reveal them, most likely because the gonotyl and the spines were embedded within the genital apparatus.
Changes in the differentiation of tegumental spines in accordance with development of worms have been reported in trematodes. In the case of
Clonorchis sinensis, larval flukes which just excysted in the duodenum had double- or triple-pointed tegumental spines at anterior half of the body, but spines gradually disappeared as the worms grew to be adults (
Fujino et al., 1979;
Lee et al., 1982). On the contrary, in larval flukes of
Fasciola hepatica, tegumental spines with a single tip metamorphosed into multipointed ones just prior to entry into the bile duct (
Bennett and Threadgold, 1975). Conversion of simple spines into more serrated ones during the parasite development was also found in
Neodiplostomum seoulense (
Lee et al., 1985) and
Paragonimus iloktsuenensis (
Lee et al., 1989). Studies on changes in surface ultrastructure of
P. summa according to developmental stages are needed.
References
- 1. Bennett CE, Threadgold LT. Fasciola hepatica: development of tegument during migration in mouse. Exp Parasitol 1975;38:38-55.
- 2. Chai JY, Chung HL, Choi MH, Sohn WM, Hong SJ, Lee SH. Surface ultrastructure of Heterophyes nocens (Trematoda: Heterophyidae). Korean J Parasitol 1992;30:75-82.
- 3. Chai JY, Guk SM, Han ET, et al. Surface ultrastructure of Metagonimus takahashii metacercariae and adults. Korean J Parasitol 2000;38:9-15.
- 4. Chai JY, Kang YJ, Choi SY, Guk SM, Yu JR, Lee SH. Surface ultrastructure of Metagonimus miyatai metacercariae and adults. Korean J Parasitol 1998b;36:217-225.
- 5. Chai JY, Kim IM, Seo M, et al. A new focus of Heterophyes nocens, Pygidiopsis summa, and other intestinal flukes in a coastal area of Muan-gun, Chollanam-do. Korean J Parasitol 1997;35:233-238.
- 6. Chai JY, Lee SH. Intestinal trematodes of humans in Korea: Metagonimus, heterophyids and echinostomes. Korean J Parasitol 1990;28(suppl.):103-122.
- 7. Chai JY, Seo BS, Lee SH, Hong ST. Growth and development of Pygidiopsis summa in rats and mice with a supplementary note on its morphological characters. Korean J Parasitol 1986;24:55-62.
- 8. Chai JY, Song TE, Han ET, et al. Two endemic foci of heterophyids and other intestinal fluke infections in southern and western coastal areas in Korea. Korean J Parasitol 1998a;36:155-161.
- 9. Chun Sk. A study on some trematodes whose intermediate hosts are brackish water fish (II) The life history of Pygidiopsis summus, the intermediate host of which is Mugil cephalus. Bull Busan Fish Coll 1963;5:1-5.
- 10. Fujino T, Ishii Y, Choi DW. Surface ultrastructure of the tegument of Clonorchis sinensis newly excysted juveniles and adult worms. J Parasitol 1979;65:579-590.
- 11. Hong SJ, Chai JY, Lee SH. Surface ultrastructure of the developmental stages of Heterophyopsis continua (Trematoda: Heterophyidae). J Parasitol 1991;77:613-620.
- 12. Koie M. Stereoscan studies of cercariae, metacercariae, and adults of Cryptocotyle lingua (Creplin, 1825) Fischoeder, 1903 (Trematoda: Heterophyidae). J Parasitol 1977;63:835-839.
- 13. Lee SH, Hong SJ, Chai JY, Seo BS. Studies on intestinal trematodes in Korea XV. Tegumental ultrastructure of Fibricola seoulensis according to developmental stages. Seoul J Med 1985;26:52-63.
- 14. Lee SH, Hong ST, Seo BS. A study on the fine tegumental structures of the metacercaria and juvenile stages of Clonorchis sinensis. Korean J Parasitol 1982;20:123-132.
- 15. Lee SH, Seo BS, Chai JY, Hong SJ. Study on Metagonimus yokogawai (Katsurada, 1912) in Korea VII. Electron microscpic observation on the tegumental structure. Korean J Parasitol 1984;22:1-10. (in Korean).
- 16. Lee SH, Kim SJ, Chai JY, Shon WM. Tegumental ultrastructure of Paragonimus iloktsuenensis according to the developmental stages. Korean J Parasitol 1989;27:57-66.
- 17. Onji Y, Nishio T. On the trematodes whose intermediate host is brackish water fishes. Chiba Igaku Semmon Gakko Zasshi 1916;81 & 82:229-249. (in Japanese).
- 18. Seo BS, Hong ST, Chai JY. Studies on intestinal trematodes in Korea III. Natural human infection of Pygidiopsis summa and Heterophyes heterophyes nocens. Seoul J Med 1981a;22:228-235.
- 19. Seo BS, Hong ST, Chai JY, Cho SY. Studies on intestinal trematodes in Korea IV. Geographical distribution of Pygidiopsis and Heterophyes metacercariae. Seoul J Med 1981b;22:236-242.
- 20. Sohn WM, Han GG, Kho WG, Chai JY, Lee SH. Infection status with the metacercariae of heterophyid flukes in the brackish water fish from Haenam-gun, Chollanam-do, Korea. Korean J Parasitol 1994;32:163-169. (in Korean).
- 21. Takahashi S. On the eggs of Stellantchasmus falcatus and Pygidiopsis summus found in human stools. Okayama Igakkai Zasshi 1929;41:1502-1513. (in Japanese).
- 22. Taraschewski H. Die Trematoden der Gattung Heterophyes Taxonomie, biologie, epidemiologie. 1984, Germany. Doctoral Dissertation to Hohenheim University.
- 23. Yokogawa M, Sano M, Itabashi T, Kachi S. Studies on the intestinal flukes II. Epidemiological studies on heterophyid trematodes of man in Chiba Prefecture. Jap J Parasitol 1965;14:577-585.
Figs. 1-7Scanning electron micrographs of Pygidiopsis summa adults (ventral view). Fig. 1. A whole adult fluke, showing its calabash form with oral and ventral suckers and the genital apparatus. Fig. 2. Tegument around the oral sucker. Note the distribution of one pair of small type I sensory papillae (arrowheads) and two pairs of large type I sensory papillae on the anterior part of the lip of the oral sucker. On the posterior part of the lip, a pair of type II sensory papillae are seen medially. Fig. 3. Tegument between oral and ventral suckers, showing dense distribution of tegumental spines, which are long painting brush-shape and have 14-16 digits. Fig. 4. Tegument around the ventral sucker and genital apparatus. Note that sperms are escaping from or entering into the genital pore. Fig. 5. Magnification of the tegumental spines in Fig. 3. Fig. 6. Tegument just anterior to the genital apparatus, showing short broom brush-shaped tegumental spines, having 14-16 digits. Fig. 7. Tegument on the posterior body surface, showing a uni-ciliated type I sensory papilla and several spade-shaped tegumental spines with 5-8 digits.
Figs. 8-14.Scanning electron micrographs of Pygidiopsis summa adults (dorsal view). Fig. 8. A whole adult fluke, showing numerous tegumental spines on the body surface, except around the posterior end of the body. Fig. 9. Tegument on the antero-lateral body surface, showing protruded cytoplasmic processes, each with a short tegumental spine having 15-17 digits. Fig. 10. Tegument on the mid-lateral body surface, showing a uni-ciliated sensory papilla and short wide tegumental spines with 19-21 digits. Fig. 11. Tegument on another mid-lateral body surface, showing protruded cytoplasmic processes, each with a spade-shaped tegumental spine having 12-14 digits. Fig. 12. Tegument on the mid-posterior body surface, showing protruded cytoplasmic processes, each with a bear claw-shaped tegumental spine having 4-5 digits. Fig. 13. Tegument on the postero-lateral body surface. Spines are sparsely distributed and claw-shaped with 3-4 digits. Fig. 14. Tegument near the posterior end of the body, wrinkled and devoid of spines.
Citations
Citations to this article as recorded by

- Ultra-structure, genetic characterization and Immunological approach of fish borne zoonotic trematodes (Family: Heterophyidae) of a redbelly tilapia
Mai A. Salem, Olfat A. Mahdy, Reem M. Ramadan
Research in Veterinary Science.2024; 166: 105097. CrossRef - Body Surface Ultrastructure as a Main Morphological Criterion for Distinguishing Adult Trematode Metagonimus suifunensis
Polina Shumenko, Yulia Tatonova, Mikhail Shchelkanov
Biology.2024; 13(11): 942. CrossRef - Anthelmintic potential of sulphonamides and Cucurbita pepo seeds extract on Heterophyes heterophyes experimentally infected mice
Dalia S. Ashour, Fetouh A. Deyab, Kamal F. Eliwa, Samy I. El-Kowrany
Journal of Parasitic Diseases.2023; 47(4): 697. CrossRef - Pygidiopsis cambodiensis n. sp. (Digenea: Heterophyidae) from experimental hamsters infected with metacercariae in mullets from Cambodia
Woon-Mok Sohn, Deok-Gyu Kim, Bong-Kwang Jung, Jaeeun Cho, Jong-Yil Chai
Parasitology Research.2016; 115(1): 123. CrossRef - Topography and ultrastructure of the tegument of Deropristis inflata Molin, 1859 (Digenea: Deropristidae), a parasite of the European eel Anguilla anguilla (Osteichthyes: Anguillidae)
Jean-José Filippi, Yann Quilichini, Bernard Marchand
Parasitology Research.2013; 112(2): 517. CrossRef - Topography and ultrastructure of the tegument of Lecithochirium musculus (digenea: Hemiuridae), a parasite of the European eel Anguilla anguilla (osteichthyes: Anguillidae)
Jean‐José Filippi, Yann Quilichini, Joséphine Foata, Bernard Marchand
Journal of Morphology.2012; 273(4): 361. CrossRef - Redescription of Parspina argentinensis (Szidat, 1954) (Digenea: Cryptogonimidae) from freshwater fishes (Pimelodidae) in the basins of the Paraná and La Plata Rivers, Argentina, with comments on P. bagre Pearse, 1920
Margarita C. Ostrowski de Núñez, Nathalia J. Arredondo, Irene L. Doma, Alicia A. Gil de Pertierra
Systematic Parasitology.2011; 78(1): 27. CrossRef - Two new species of Parspina Pearse, 1920 (Digenea: Cryptogonimidae) from freshwater fishes (Gymnotiformes) of the Paraná River basin in Argentina
Margarita C. Ostrowski de Núñez, Nathalia J. Arredondo, Alicia A. Gil de Pertierra
Systematic Parasitology.2011; 80(1): 67. CrossRef - Tegumental Ultrastructure of Adult Gynaecotyla squatarolae (Digenea: Microphallidae)
Do-Seon Lim, Ki-Ju Choi, Sang-Mee Guk, Jong-Yil Chai, Il-Yong Park, Yun-Kyu Park, Min Seo
The Korean Journal of Parasitology.2008; 46(2): 87. CrossRef - TAXONOMIC STATUS, REDESCRIPTION, AND SURFACE ULTRASTRUCTURE OF ASCOCOTYLE (PHAGICOLA) PINDORAMENSIS N. COMB. (DIGENEA: HETEROPHYIDAE)
Susana Balmant Emerique Simões, Tomáš Scholz, Helene Santos Barbosa, Cláudia Portes Santos
Journal of Parasitology.2006; 92(3): 501. CrossRef - REDESCRIPTION AND SURFACE ULTRASTRUCTURE OF PYGIDIOPSIS MACROSTOMUM (DIGENEA: HETEROPHYIDAE)
S. B E. Simões, H. S. Barbosa, C. P. Santos
Journal of Parasitology.2005; 91(4): 931. CrossRef