Abstract
The effect of a secretory proteinase from the pathogenic amoebae Acanthamoeba castellanii on host's defense-oriented or regulatory proteins such as immunoglobulins, interleukin-1, and protease inhibitors was investigated. The enzyme was found to degrade secretory immunoglobulin A (sIgA), IgG, and IgM. It also degraded interleukin-1α (IL-1α) and IL-1β. Its activity was not inhibited by endogenous protease inhibitors, such as α2-macroglobulin, α1-trypsin inhibitor, and α2-antiplasmin. Furthermore, the enzyme rapidly degraded those endogenous protease inhibitors as well. The degradation of host's defense-oriented or regulatory proteins by the Acanthamoeba proteinase suggested that the enzyme might be an important virulence factor in the pathogenesis of Acanthamoeba infection.
-
Key words: Acanthamoeba, protease inhibitors, immunoglobulins, interleukin-1
INTRODUCTION
Acanthamoeba keratitis is a progressive sight-threatening corneal disease caused by pathogenic, free-living amoebae
Acanthamoeba species. The infection is highly resistant to topical and systemic therapy, leading to serious visual impairment or even loss of vision. The pathogenesis of
Acanthamoeba occurs in a multistep process that begins with the binding of the trophozoites to the corneal epithelium (
Niederkorn et al., 1992;
Yang et al., 1997). Following binding on the corneal epithelial surface, trophozoites invade deeper regions of the corneal epithelium and penetrate the stroma, resulting in necrosis, edema, disruption of the stromal lamellae, and an intense polymorphonuclear inflammatory response (
Mathers et al., 1987). During this process,
Acanthamoeba trophozoites elicit several cytopathic factors that induce considerable corneal damage and allow the parasite to invade the stroma (
He et al., 1990;
Mitro et al., 1994;
Leher et al., 1998a;
Na et al., 2001).
Proteinases of parasites have been known to be important virulence factors in the pathogenicity of parasitic infections either by inducing tissue damage and facilitating invasion or by empowering the parasites to salvage metabolisms from host proteins (
McKerrow, 1989). As other parasitic protozoa, pathogenic
Acanthamoeba trophozoites produce a variety of proteinases (
He et al., 1990;
Mitro et al., 1994;
Mitra et al., 1995;
Park and Song, 1996;
Cho et al., 2000;
Kong et al., 2000;
Na et al., 2001). Although the precise role of these proteinases in the pathogenesis of corneal infection of
Acanthamoeba is not clear at present, some evidence suggests that the pathogenic roles of the proteinases as important virulence factors in
Acanthamoeba keratitis. The secretory products from trophozoites of
Acanthamoeba have been shown to have collagenolytic activity, which damages collagen shield in an in vitro assay and rat cornea in vivo (
He et al., 1990;
Mitro et al., 1994). Furthermore,
Acanthamoeba proteinases can break down a number of host proteins, including major structural proteins such as collagen, fibronectln and laminin (
Park and Song, 1996;
Cho et al., 2000;
Kong et al., 2000;
Na et al., 2001). Importantly, intrastromal injection of culture medium and purified proteinase of
A. castellanii into the corneas of rabbits produced characteristic ring infiltrates and corneal lesions that clinically and histopathologically resembled those found in patients with
Acanthamoeba keratitis (
He et al., 1990;
Na et al., 2001). These results partially suggest that
Acanthamoeba proteinases are closely associated with the pathogenesis of
Acanthamoeba by facilitating penetration of the parasites into the corneal stroma.
However, to elucidate the pathogenic role of the Acanthamoeba proteinase in Acanthamoeba infection more definitely, more detailed studies on the pathogenic mechanism of the proteinase are needed. In this study, we investigated the effect of the Acanthamoeba proteinase on the host's defense-oriented or regulatory proteins.
MATERIALS AND METHODS
Organism and culture condition
Acanthamoeba castellanii isolated from a patient with
Acanthamoeba keratitis was grown axenically at 30℃ in peptone-yeast extract-glucose (PYG) medium as described previously (
Silvany et al., 1990).
Purification of secretory proteinase of Acanthamoeba
A 12 kDa secretory serine proteinase of
A. castellanii was purified from the culture supernatant as described previously (
Na et al., 2001). The purified enzyme was examined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE was performed by the method of Laemmli (
1970) using a 12% (w/v) polyacrylamide gel. The protein concentration was measured by the method of Lowry et al. (
1951).
The enzyme activity was determined spectrophotometrically following the digestion of azocasein as the substrate. To the enzyme solution, 300 µl of 2% azocasein in 50 mM Tris-HCl buffer (pH 8.5) was added and incubated at 37℃ for 2 h. The reaction was then stopped by adding 700 µl of ice-cold 10% trichloroacetic acid (TCA). The precipitated protein was removed by centrifugation at 10,000 rpm for 3 min and 700 µl of the resulting supernatant was mixed with 600 µl of 1 N NaOH. The proteolytic activity was estimated by measuring absorbance of the above mixture at 440 nm. One unit of enzyme activity was defined as the amount of enzyme needed to increase OD440 to 0.1 under the above condition.
Degradation of immunoglobulins and protease inhibitors
Human immunoglobulins (Igs), secretory IgA (slgA), IgG, and IgM, and protease inhibitors including α2-macroglobulin, α1-trypsin inhibitor, and α2-antiplasmin, were purchased from Sigma. These proteins (each 10 µg) were incubated with purified enzyme (0.1 µg) in 50 mM Tris-HCl buffer (pH 8.5) at 37℃ for indicated time. The reactions were stopped by adding an equal volume of denaturing sample buffer followed by boiling the mixture for 3 min. SDS-PAGE was performed by the method described above.
Inhibition of proteinase activity by protease inhibitors
Inhibition of the proteinase activity by human endogenous protease inhibitors such as α2-macroglobulin, α1-trypsin inhibitor, and α2-antiplasmin was examined at an enzyme/inhibitor molar ratio of 1:1 to 1:10 in 50 mM Tris-HCl buffer (pH 8.5) at 37℃ for 30 min to 24 h. After then, residual proteinase activity was determined using azocasein as the substrate as described above. All the experiments were performed triplicate and means ± standard deviations were calculated.
Degradation of interleukin-1 (IL-1)
Human recombinant IL-1α and IL-lβ (each 0.2 µg) (Sigma) were incubated with the purified enzyme (0.1 µg) at 37℃ for 0.5 and 1 h, respectively. Following the incubation, SDS-PAGE on 15% polyacrylamide gel was performed. Proteins on the gel were electrophoretically transferred onto a nitrocellulose membrane (Bio-Rad). After then, the membrane was blocked in PBST [0.05% Tween 20 in phosphate buffered saline (PBS), pH 7.4] containing 3% skim milk for 1 h at room temperature, and then incubated with 1:1,000 diluted goat anti-human IL-1α IgG and goat anti-human IL-lβ IgG (Sigma) for 2 h at room temperature, respectively. After washing three times with PBST, the membrane was incubated with peroxidase-conjugated anti-goat IgG (Sigma) for 2 h at room temperature. After additional three washing with PBST, the membrane was incubated in a freshly prepared substrate solution (4 mg/ml of 3, 3'-diaminobenzidine, 0.01% of hydrogen peroxide in 0.1 M PBS, pH 7.4) for 10 min at room temperature. The reaction was stopped by washing the membrane with distilled water several times.
RESULTS
Degradation of immunoglobulins
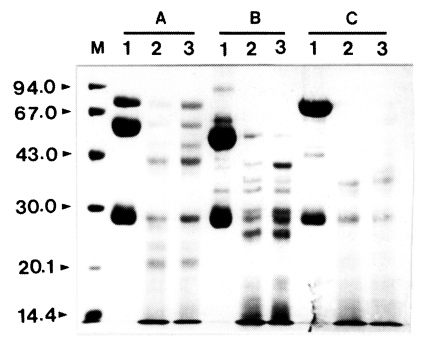
To investigate the proteolytic degradation of human immunoglobulins by
Acanthamoeba proteinase, the mixtures of the purified enzyme and slgA, IgG, or IgM were analyzed by SDS-PAGE after different incubation periods. The enzyme degraded these immunoglobulins in a time-dependent manner (
Fig. 1). When cleaved by the proteinase, slgA resulted in the generation of smaller fragments with approximate molecular weights of about 49, 41, and 21 kDa as well as other minor fragments. The similar results were also observed with IgG and IgM. IgG was digested by the enzyme and the products of approximate molecular weights of 34, 36, and 40 kDa were observed. The digestion pattern of IgM by the proteinase was quite different from those of slgA and IgG. When compared to the digestion patterns of slgA and IgG, the digested fragments of IgM were thoroughly diffused between 30 and 43 kDa, and the bands were difficult to visualize.
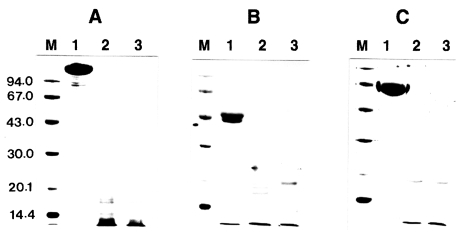
The proteolytic activity of the enzyme was not affected by different types of protease inhibitors including α2-macroglobulin, α1-trypsin inhibitor, and α2-antiplasmin even at an excess concentration of inhibitors. The enzyme was not inhibited significantly even after an extended incubation period of longer than 24 h (data not shown). These results indicated that the inhibitors did not affect the activity of the proteinase. To account for this lack of inhibition, incubation mixtures of proteinase and protease inhibitor were analyzed by SDS-PAGE after 0.5 and 1 h of incubation at 37℃.
Fig. 2. shows that all the protease inhibitors were completely degraded by the enzyme.
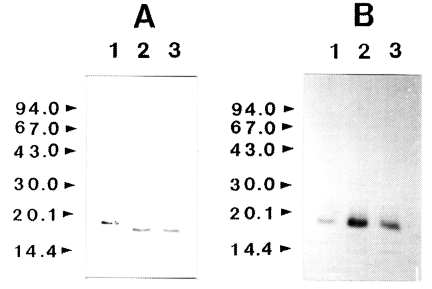
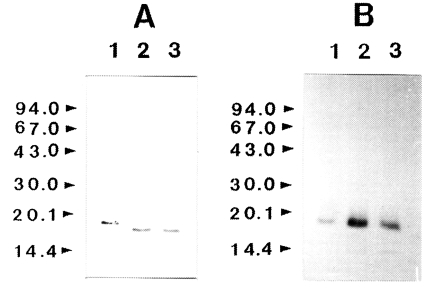
To determine whether IL-1 was susceptible to proteolytic cleavage by the proteinase, human IL-1α and IL-lβ (0.2 µg) was incubated with the purified enzyme and the products were analyzed by SDS-PAGE and then by immunoblot, respectively. The results of a representative experiment are shown in
Fig. 3. Both the IL-1α and IL-lβ were cleaved, producing lower-molecular weight fragments. The 17 kDa IL-1α was degraded by the enzyme and additional bands with molecular mass of approximately 16 kDa was produced. Fragments with molecular mass lower than 15 kDa were also produced. In the case of IL-lβ, the cleaved product of approximate molecular weight of 14.5 kDa was observed. The results of these experiments indicated that the recombinant IL-1α and IL-1β were susceptible to proteolytic cleavage by the proteinase.
DISCUSSION
The ocular surface is regularly exposed to the external environment and potential pathogens, however, is protected by vigorous defense mechanisms that would promptly eliminate the pathogens. These defense mechanisms are largely provided by tear film that forms the first line of defense between the external environment and the ocular surface. Tears contain variety of humoral defense components such as lactoferrin, lysozyme, and lactoperoxidase. In addition to innate humoral defense mechanisms, tears also contain various other components that act as defense-oriented or regulatory molecules (
Hazlett and Berk, 1989;
Fullard and Snyder, 1990).
One of them is immunoglobulins that may play a major role as a host defense mechanism to prevent or modulate microbial adhesion, invasion, and infectivity (
Allansmith et al., 1979;
Wells and Hazlett, 1985). In the present study, the effect of
A. castellanii proteinase on immunoglobulins was investigated. The purified enzyme of
A. castellanii degraded slgA. slgA is the major immunoglobulin isotype present in normal tears where it constitutes a front line of defense against microorganisms primarily as an immunologic barrier by preventing adherence and adsorption of microbes (
Gachon et al., 1982;
Gregory and Allansmith, 1986;
Mestecky et al., 1986;
Masinick et al., 1997). It may also prevent colonization by neutralizing microbial pathogens within corneal epithelial cells. Inhibition of microorganism's adherence to the ocular surface would be advantageous, because this might diminish the host inflammatory cell response to eliminate microorganisms from the cornea, in turn leading to less innocent bystander destruction of the cornea tissue. Similar to most other ocular pathogens, the first step of
Acanthamoeba infection is adhesion of trophozoites to corneal epithelial surfaces (
Niderkorn et al., 1992;
Yang et al., 1997). Inhibiting the binding of
Acanthamoeba trophozoites to the corneal epithelium may be a highly effective mechanism for preventing corneal infection. In fact, the orally immunized animals produced IgA antibodies specific for
Acanthamoeba antigens, and they inhibited the adhesion of trophozoites to corneal epithelial cells in vitro (
Leher et al., 1998b;
Leher et al., 1999). Therefore, cleavage of slgA by proteinases would presumably enhance the ability of the organism to survive on corneal surfaces. The proteinase of
A. castellanii was also able to degrade IgG and IgM. IgG and IgM are involved in the complement-dependent killing mechanism such as opsonization and phagocytosis, in microbial infections. Although repeated intramuscular immunizations of
Acanthamoeba antigens induced
Acanthamoeba-specific serum IgG and delayed-type hypersensitivity (DTH) responses, they failed to protect against an ocular challenge by
Acanthamoeba trophozoites (
Alizadeh et al., 1995;
van Klink et al., 1997). Moreover, repeated ocular infections do not induce protective immunity (
Alizadeh et al., 1995;
van Klink et al., 1997). Failure of protective immunity against
Acanthamoeba infection may be partially explained by the fact that
Acanthamoeba proteinase degraded immunoglobulins. Degradation of immunoglobulins by the
Acanthamoeba proteinase may result in a decrease of host immune activity to
Acanthamoeba and may render hosts more vulnerable to subsequent opportunistic infections.
We also investigated the effect of
A. castellanii proteinase on several protease inhibitors. Protease inhibitors have been identified in tears, and their amounts are increased in the case of infections or in corneal ulcerations (
Stanifer et al., 1983). These endogenous protease inhibitors are involved in critical regulation of various cascade systems, including the complement-dependent defense system, the kallikrein-kinin system, the fibrinolytic and clotting system, and inflammation reactions. These cascades may be highly enhanced when the inhibitors are inactivated. Furthermore, protease inhibitors of hosts may act as defense-oriented molecules that inhibit proteinases of pathogens. As shown in this study, the endogenous protease inhibitors, such as α2-macroglobulin, α2-antiplasmin, and α1-antitrypsin, did not inhibit the activity of
A. castellanii proteinase, and the inhibitors themselves were completely degraded.
Acanthamoeba proteinases are known to be important virulence factors that induce tissue damage and facilitate invasion of the amoeba into the corneal stroma (
He et al., 1990;
Mitro et al., 1994;
Na et al., 2001). Therefore, degradation of various protease inhibitors may not only enhance the pathogenicity of
Acanthamoeba, but also may affect the activity of endogenous proteinases in the cornea, resulting in more severe pathological consequences, such as tissue destruction, edema formation, and pain. Actually, when the purified enzyme was injected intrastromally to rabbit cornea, characteristic ring infiltration and corneal lesions that clinically resembled those in
Acanthamoeba keratitis were observed (
Na et al., 2001). These lesions were significantly inhibited by the protease inhibitor, phenylmethyl sulfonylfluoride. This further lends support to this hypothesis.
The inability of corneal infections to induce primary cell-mediated immune responses is due to the absence of resident antigen presenting cells (APCs) in the central cornea. Langerhans cells (LCs), known to be major APCs in the cornea, are uniquely distributed in the peripheral region of the cornea. Peripheral LCs migrate into the central cornea either by intracorneal injection of IL-1 or by instillation of sterile latex beads prior to infection with
Acanthamoeba trophozoites, and they produce a remarkable resistance to infection (
van Klink et al., 1993;
van Klink et al., 1997). Although a definite mechanism of the protective effect is not clear yet, this suggests that LCs play important roles in immunological protection against
Acanthamoeba infection. It is well known that potential pathogens and antigens would be phagocytosed by corneal epithelial cells, which in turn would elaborate increased quantities of IL-1, a molecule which plays a central role in the induction, progression, and maintenance of an inflammatory response (
Dinarello, 1994). The soluble IL-1 would then act as a potent chemoattractant for peripheral LCs residing in the limbus. LCs would migrate in a unidirectional manner and accumulate at the site of antigen deposition and IL-1 synthesis, where they encounter the activated corneal epithelial cells. The corneal epithelial cells may display the partially processed antigen on their cell membranes for delivery to the infiltrating LCs. Although LCs itself did not provoke primary cell-mediated immunity, further antigen processing occurred within the LCs, which would then migrate to regional lymphoid tissues for the final stage of antigen presentation to T and B lymphocytes.
The enzyme of Acanthamoeba degraded both IL-lα and IL-lβ. Degradation of IL-1 by the enzyme might not induce migration of LCs into the central cornea, thereby resulting in failure of primary cell-mediated immunity. Consequently, it might enhance the survival of Acanthamoeba against the hosts' systemic immune response and make symptoms more severe.
In conclusion, in the present study, we investigated a potent proteolytic action of Acanthamoeba proteinase on the host's defense-oriented or regulatory proteins, including immunoglobulins, interleukin-1, and several protease inhibitors. Acanthamoeba proteinase could degrade these proteins. These results suggest that Acanthamoeba proteinase is a potential virulence factor that could enhance the ability of Acanthamoeba to survive against the host's defense mechanism and render hosts more vulnerable to subsequent opportunistic infections.
Notes
-
This study was supported by a grant of the National Institute of Health, Ministry of Health and Welfare, Republic of Korea (NIH-348-6111-215).
References
- 1. Alizadeh H, He YG, McCulley JP, et al. Successful immunization against Acanthamoeba keratitis in a pig model. Cornea 1995;14:180-186.
- 2. Allansmith M, de Ramus A, Maurice D. The dynamics of IgG in the cornea. Invest Ophthalmol Vis Sci 1979;18:947-955.
- 3. Cho JH, Na BK, Kim TS, Song CY. Purification and characterization of an extracellular serine proteinase from Acanthamoeba castellanii. IUBMB Life 2000;50:209-214.
- 4. Dinarello CA. The biological properties of interleukin-1. Eur Cytokine Netw 1994;5:517-531.
- 5. Fullard RJ, Snyder C. Protein levels in nonstimulated and stimulated tears of normal human subjects. Invest Ophthalmol Vis Sci 1990;31:1119-1126.
- 6. Gachon AM, Richard J, Dastugue B. Human tears: Normal protein pattern and individual protein determinations in adults. Curr Eye Res 1982;2:301-308.
- 7. Gregory RL, Allansmith MR. Naturally occurring IgA antibodies to ocular and oral microorganisms in tears, salive and colostrum: Evidence for a common mucosal immune system and local immune response. Exp Eye Res 1986;43:739-749.
- 8. Hazlett LD, Berk RS. Kinetics of immunoglobulin appearance at the ocular surface. Reg Immunol 1989;2:294-299.
- 9. He YG, Niederkorn JY, McCulley JP, et al. In vivo and in vitro collagenolytic activity of Acanthamoeba castellanii. Invest Ophthalmol Vis Sci 1990;31:2235-2240.
- 10. Kong HH, Kim TH, Chung DI. Purification and characterization of a secretory serine proteinase of Acanthamoeba healyi isolated from GAE. J Parasitol 2000;86:12-17.
- 11. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-685.
- 12. Leher H, Silvany R, Alizadeh H, Huang J, Niederkorn JY. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii. Infect Immun 1998a;66:5-10.
- 13. Leher H, Alizadeh H, Taylor WM, et al. Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Invest Ophthalmol Vis Sci 1998b;39:2666-2673.
- 14. Leher H, Zaragoza F, Taherzadeh S, Alizadeh H, Niederkorn JY. Monoclonal IgA antibodies protect against Acanthamoeba keratitis. Exp Eye Res 1999;69:75-84.
- 15. Lowry OH, Rosebrugh NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265-275.
- 16. Masinick SA, Montgomery CP, Montgomery PC, Hazlett LD. Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Invest Ophthalmol Vis Sci 1997;38:910-918.
- 17. Mathers W, Stevens G Jr, Rodrigues M, et al. Immunopathology and electron microscopy of Acanthamoeba keratitis. Am J Ophthalmol 1987;103:626-635.
- 18. McKerrow JH. Parasite proteases. Exp Parasitol 1989;68:111-115.
- 19. Mestecky J, Russell MW, Jackson S, Brown TA. The human IgA system: A reassessment. Clin Immunol Immunopathol 1986;40:105-114.
- 20. Mitra MM, Alizadeh H, Gerard RD, Niederkorn JY. Characterization of a plasminogen activator produced by Acanthamoeba castellanii. Mol Biochem Parasitol 1995;73:157-164.
- 21. Mitro K, Bhagarathiammai A, Zhou OM, et al. Partial characterization of the proteolytic secretions of Acanthamoeba polyphaga. Exp Parasitol 1994;78:377-385.
- 22. Na BK, Kim JC, Song CY. Characterization and pathogenetic role of proteinase from Acanthamoeba castellanii. Microb Pathog 2001;30:39-48.
- 23. Niderkorn JY, Ubelaker JE, McCulley JP, et al. Susceptibility of corneas from various animal species to in vitro binding and invasion by Acanthamoeba castellanii. Invest Ophthalmol Vis Sci 1992;33:104-112.
- 24. Park KW, Song CY. Purification and characterization of a serine proteinase from Acanthamoeba culbertsoni. J Biochem Mol Biol 1996;29:455-461.
- 25. Silvany RE, Dougherty JM, McCulley JP, Wood TS, Bowman RW, Moore MB. The effect of currently available contact lens disinfection systems on Acanthamoeba castellanii and Acanthamoeba polyphaga. Ophthalmology 1990;97:286-290.
- 26. Stanifer RM, Andrews SS, Kretzer FL. In Anderson R.E. ed, Tear film. Biochemistry of the Eye. 1983, San Francisco. American Academy of Ophthalmology; pp 7-2 Manuals Program.
- 27. van Klink F, Alizadeh H, He Y, et al. The role of contact lens, trauma, and Langerhans cells in a Chinese hamster model of Acanthamoeba keratitis. Invest Ophthal Vis Sci 1993;34:1937-1944.
- 28. van Klink F., Leher H, Jager MJ, et al. Systemic immune response to Acanthamoeba keratitis in the Chinese hamster. Ocul Immunol Inflamm 1997;5:235-244.
- 29. Wells PA, Hazlett LD. Immunocytochemical localization of immunoglobulins at the corneal surface of the mouse. Exp Eye Res 1985;40:779-796.
- 30. Yang ZT, Cao ZY, Panjwani N. Pathogenesis of Acanthamoeba keratits-carbohydrate-mediated host-parasite interactions. Infect Immun 1997;65:439-445.
Fig. 1Effect of Acanthamoeba castellanii proteinase on human immunoglobulins. Immunoglobulins (10 µg) were incubated with the purified enzyme at 37℃ for 0.5 and 1 h, respectively. After incubation, samples were heated at 100℃ for 3 min and analyzed SDS-PAGE. (A) Secretory immunoglobulin A. (B) Immunoglobulin G. (C) Immunoglobulin M. Lane 1, no proteinase; Lanes 2-3; incubated with the proteinase (0.1 µg) at 37℃ for 0.5 and 1 h,

Fig. 2Effect of Acanthamoeba castellanii proteinase on human protease inhibitors. Protease inhibitors, α2-macroglobulin, α1-trypsin inhibitor, or α2-antiplasmin (10 µg), were incubated with the purified proteinase at 37℃ for 0.5 and 1 h, respectively. After incubation, samples were heated at 100℃ for 3 min and analyzed by SDS-PAGE. (A) α2-macroglobulin. (B) α1-trypsin inhibitor. (C) α2-antiplasmin. Lane 1, no proteinase; Lanes 2-3; incubated with the proteinase (0.1 µg) at 37℃ for 0.5 and 1 h, respectively.
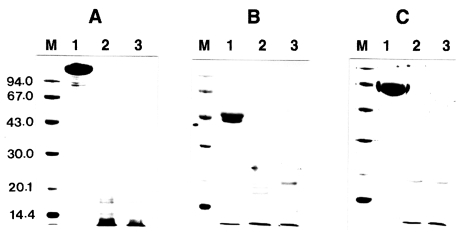
Fig. 3Effect of Acanthamoeba castellanii proteinase on human IL-lα and IL-lβ. Recombinant human IL-lα and IL-lβ (0.2 µg) was incubated with proteinase at 37℃ for 0.5 and 1 h, respectively. After incubation, samples were heated at 100℃ for 3 min and analyzed by immunoblot. (A) IL-lα. (B) IL-lβ. Lane 1, no proteinase; Lanes 2-3; incubated with the proteinase (0.1 µg) at 37℃ for 0.5 and 1 h, respectively.