Abstract
Genotyping of Toxoplasma gondii has been performed in 23 PCR positive blood samples from stray cats in Korea. We used 2 separate PCR-restriction fragment length polymorphism (RFLP) patterns of SAG2 gene, amplifying the 5'and 3'ends of the locus. The results revealed that all samples belonged to the type I clonal lineage. Although T. gondii organisms were not isolated from the samples, the results of the present study represent that stray cats with T. gondii infection should be seriously concerned in our environment. Adequate and continuous control programs of stray cats are needed to reduce the risk of transmission of T. gondii as a zoonotic infection threatening the public health.
-
Key words: Toxoplasma gondii, PCR-RFLP, stray cat, SAG2
Toxoplasma gondii is an obligate intracellular protozoan that can infect both humans and warm-blooded animals. Among them, cats are the only animal that excretes oocysts into the environment [
1]. As recently reported, 47.2% out of 106 stray cats [
2] and 13.2% out of 174 stray cats [
3] were
T. gondii positive by nested PCR assay in Korea. Stray cats have been increasing in Korea roaming residential areas, and this increased the risk of public health hazards.
T. gondii strains have been classified into 3 major genotypes (I, II, and III) according to their virulence, and among them, type I strain, such as RH, has been reported to be most virulent. This study is a follow-up of our previous study [
3] for genotyping of
T. gondii using 23 blood DNA samples of stray cats in Korea, which presented positive reactions by B1 gene amplification.
Biodiversity of
T. gondii has been reported with genetic variations among isolates from different, and even the same, phenotypes [
4]. Usually,
T. gondii is categorized as 3 genetic types (I, II, and III) based on polymorphisms in several markers, such as
SAG1,
SAG2,
SAG3,
BTUB,
GRA6, c22-8, c29-2,
PK1, and Apico genes [
5,
6]. We chose to develop a nested-PCR method based on the polymorphic
SAG2 locus, separately amplifying the 5'and 3'ends of the locus using an Accupower PCR premix Kit (Bioneer Co., Seoul, Korea), and amplifying 241 bp and 221 bp products [
7,
8]. This gene is ideally suited for rapid genotyping, as it contains multiple lineage-specific polymorphisms [
7,
9,
10].
SAG2 encodes 2 separate forms of the surface tachyzoite protein (p22), that type I (high virulent) and III (avirulent) strains share the same protein alleles, while type II strains (avirulent) have a second, distinct form [
5,
7].
Sau3A I and
Hha I restriction enzymes were used for this PCR-RFLP analysis. Digestion of the 5' amplification products with
Sau3A I distinguished allele 3 (type III: includes avirulent strains like VEG and CTG) from alleles 1 and 2 (type I: includes strains like RH and KI-1 which are highly virulent, and type II: includes avirulent strains like PLK and Beverley) [
8]. Digestion of the 3' amplification products with
Hha I distinguished allele 2 (type II strains) from alleles 1 and 3 (type I and III strains) [
7].
RH and KI-1 strains as type I, and ME49 strain as type II, were used for standardization of PCR-RFLP analysis. The representative strain as type III was not prepared because genotyping could be properly discriminated using strains mentioned above. RH strain was kindly provided from Catholic Institute of Parasitic Diseases, Catholic University, and KI-1 and ME49 strains were kindly provided from Department of Parasitology and Tropical Medicine, Seoul National University College of Medicine. RH and KI-1 strains of T. gondii were maintained in BALB/c mice by intraperitoneal inoculation in our laboratory.
PCR programs were as follows: The 5' and 3' ends of the
SAG2 locus were amplified by 5 min of denaturation at 94℃. After the first amplification of 5'-end product, this step was followed by 40 cycles, with 1 cycle consisting of 45 sec at 94℃, 30 sec at 64℃, and 30 sec at 72℃ using SAG2F4 (5'-GACCTCGAACAGGAACAC-3') and SAG2R4 (5'-GCATCAACAGTCTTCGTTGC-3') primers. In the second reaction, the primers SAG2F (5'-GAAATGTTTCAGGTTGCTGC-3') and SAG2R2 (5'-GCAAGAGCGAACTTGAACAC-3') were used, with 60℃ as the annealing temperature. The 3' end of the locus was amplified with the primers SAG2F3 (5'-TCTGTTCTCCGAAGTGACTCC-3') and SAG2R3 (5'-TCAAAGCGTGCATTATCGC-3') for the initial amplification at an annealing temperature of 62℃. In nested PCR reaction, 50 cycled amplification at annealing at 58℃ with SAG2F2 (5'-ATTCTCATGCCTCCGCTTC-3') and SAG2R (5'-AACGTTTCACGAAGGCACAC-3'). Nested PCR was performed using 10
pmol of primers and 1 µl of the first PCR product as the template. The 5' end amplification is expected to yield a product of 241 bp, while the 3' end amplification will give a product of 221 bp in all representative strain of
T. gondii and samples. All amplified fragments were purified with QIA quick gel extraction kit (Qiagen, Hilden, Germany), digested with
Sau3A I and
Hha I restricting enzymes. The restricted fragments were analyzed by 1.5% agarose gel electrophoresis (
Figs. 1,
2). Nested amplification of 5' end of the
SAG2 gene was successful in 13 of 23 (56.5%) blood DNA samples, and samples of 3' end of the
SAG2 gene was in 8 of 23 (34.8%).
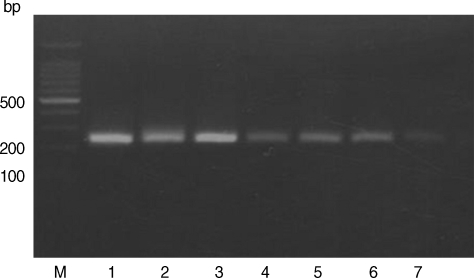
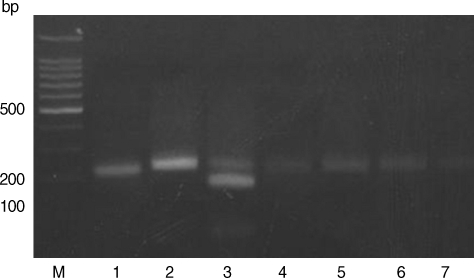
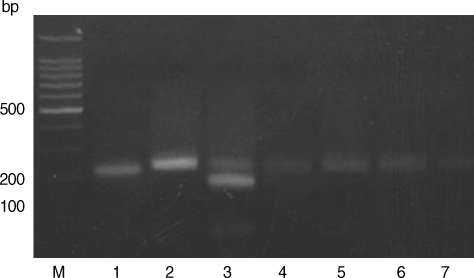
Based on the criteria defined by Howe et al. [
7] and Zakimi et al. [
10], the genotyping of
T. gondii in stray cats in Korea was established in this study. The samples spiked with either type I or type II DNA revealed RFLP patterns, with lack of digestion by
Sau3A I (
Fig. 1). The RFLP patterns of all samples showed the presence of undigested fragments at
Hha I (
Fig. 2). Therefore, all samples are categorized as type I that includes strains like RH and KI-1 which are highly virulent. The digestion patterns of
Sau3A I and
Hha I of RH and KI-1 strains were type I, and the digestion patterns of ME49 RFLP was type II. These results are similar to that of a previous report by Quan et al. [
4]. A recent population genetic study of
T. gondii in South America demonstrated high diversity but that in Southeast Asia was much less [
11]. In Korea, only 1 type of
T. gondii has been reported as described by Quan et al. [
5] and revealed in the present study to date. As Montoya et al. [
12] reported that 2 types of
T. gondii could co-exist in brains of cats, multi-type of
T. gondii may exist in variable tissues of the body. In our study, brains were not examined, and thus further studies of multiple tissues in cats are needed. In addition, free-living animals, such as stray cats and wild rodents living around the urban areas, are important for determining the epidemiological significance of
T. gondii infection, since cases of human toxoplasmosis have been reported in Korea [
13].
As increasingly large numbers of stray cats are found roaming residential areas in Korea, residents have complained of noise damage at night and the risk of public health for animals and humans. Therefore, local governments have performed TNR (trap, neuter, and return) programs to decrease the number of stray cats. Because of high risk of human toxoplasmosis, control programs of urban stray cat populations should be operated and continuously enforced. It is also necessary to isolate of T. gondii strains from stray cats in Korea and to characterize their properties in future studies.
ACKNOWLEDGEMENTS
This research was supported by a fund (4847-302-210-13, 2008) by Korea Centers for Diseases Control and Prevention.
References
- 1. Kasper LH, Buzoni-Gatel D. Some opportunistic parasitic infections in AIDS: candidiasis, pneumocytosis, cryptosporidiosis, toxoplasmosis. Parasitol Today 1998;14:150-156.
- 2. Lee JY, Lee SE, Lee EG, Song KH. Nested PCR-based detection of Toxoplasma gondii in German shepherd dogs and stray cats in South Korea. Res Vet Sci 2008;85:125-127.
- 3. Kim HY, Kim YA, Kang SW, Lee HS, Rhie HG, Ahn HJ, Nam HW, Lee SE. Prevalence of Toxoplasma gondii in stray cats of Gyeonggi-do, Korea. Korean J Parasitol 2008;46:199-201.
- 4. Sreekumar C, Hill DE, Miska KB, Vianna MCB, Yan L, Myers RL, Dubey JP. Genotyping and detection of multiple infections of Toxoplasma gondii using pyrosequencing. Int J Parasitol 2005;35:991-999.
- 5. Quan JH, Kim TY, Choi IU, Lee YH. Genotyping of a Korean isolate of Toxoplasma gondii by multilocus PCR-RFLP and microsatellite analysis. Korean J Parasitol 2008;46:105-108.
- 6. Sundar N, Cole RA, Thomas NJ, Majumdar D, Dubey JP, Su C. Genetic diversity among sea otter isolates of Toxoplasma gondii. Vet Parasitol 2008;151:125-132.
- 7. Howe DK, Honoré S, Derouin F, Sibley LD. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol 1997;35:1411-1414.
- 8. Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 1995;172:1561-1566.
- 9. Frzaeli A, Ebrahimzadeh A. A new perspective on and re-assessment of SAG2 locus as the tool for genetic analysis of Toxoplasma gondii isolates. Parasitol Res 2007;101:99-104.
- 10. Zakimi S, Kyan H, Oshiro M, Sugimoto C, Fujisaki K. PCR-based discrimination of Toxoplasma gondii from pigs at an abattoir in Okinawa, Japan. J Vet Med Sci 2006;68:401-404.
- 11. Dubey JP, Huong LT, Sundar N, Su C. Genetic characterization of Toxoplasma gondii isolates in dogs from Vietnam suggests their South American origin. Vet Parasitol 2007;146:347-351.
- 12. Montoya A, Miró G, Mateo M, Ramírez C, Fuentes I. Molecular characterization of Toxoplasma gondii isolates from cats in Spain. J Parasitol 2008;94:1044-1046.
- 13. Chai JY, Lin A, Shin EH, Oh MD, Han ET, Nam HW, Lee SH. Laboratory passage and characterization of an isolate of Toxoplasma gondii from an ocular patient in Korea. Korean J Parasitol 2003;41:147-154.
Fig. 1Restriction patterns of PCR products amplified from blood DNA of stray cats with T. gondii strains. 5' SAG2 amplification products were digested with Sau3AI in 2% agarose. M, 100 bp ladder; Lane 1, RH (type I); Lane 2, KI-1 (type I); Lane 3, ME49 (type II); Lanes 4-7, Blood DNA extracted from stray cats.
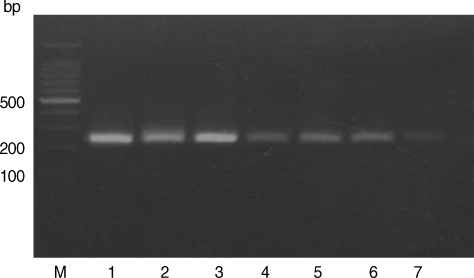
Fig. 2Restriction patterns of PCR products amplified from blood DNA of stray cats with T. gondii strains. 3' SAG2 amplification products were digested with HhaI in 2% agarose. M, 100 bp ladder; Lane 1, RH (type I); Lane 2, KI-1 (type I); Lane 3, ME49 (type II); Lanes 4-7, blood DNA extracted from stray cats.
Citations
Citations to this article as recorded by

- An outbreak of toxoplasmosis in squirrel monkeys (Saimiri sciureus) in South Korea
Hanseul Oh, Kyung‐Yeon Eo, Sanjeev Gumber, Jung Joo Hong, C‐Yoon Kim, Hyun‐Ho Lee, Young‐Mok Jung, Jin Kim, Gyu‐Whan Whang, Ji‐Min Lee, Yong‐Gu Yeo, Bokyeong Ryu, Ji‐Sook Ryu, Seul‐Kee Lee, Ukjin Kim, Sin‐Geun Kang, Jae‐Hak Park
Journal of Medical Primatology.2018; 47(4): 238. CrossRef - Geographical distribution of Toxoplasma gondii genotypes in Asia: A link with neighboring continents
P. Chaichan, A. Mercier, L. Galal, A. Mahittikorn, F. Ariey, S. Morand, F. Boumédiène, R. Udonsom, A. Hamidovic, J.B. Murat, Y. Sukthana, M.L. Dardé
Infection, Genetics and Evolution.2017; 53: 227. CrossRef - A high seroprevalence ofToxoplasma gondiiantibodies in a population of feral cats in the Western Cape province of South Africa
Kenneth Hammond-Aryee, Monika Esser, Lesley van Helden, Paul van Helden
Southern African Journal of Infectious Diseases.2015; 30(4): 141. CrossRef - Prevalence and genetic characterization of Toxoplasma gondii infection in bats in southern China
H.H. Jiang, S.Y. Qin, W. Wang, B. He, T.S. Hu, J.M. Wu, Q.S. Fan, C.C. Tu, Q. Liu, X.Q. Zhu
Veterinary Parasitology.2014; 203(3-4): 318. CrossRef - Prevalence and molecular characterizations of Toxoplasma gondii and Babesia microti from small mammals captured in Gyeonggi and Gangwon Provinces, Republic of Korea
Sung-Hee Hong, Sang-Eun Lee, Young-Il Jeong, Heung-Chul Kim, Sung-Tae Chong, Terry A. Klein, Jin-Won Song, Se Hun Gu, Shin-Hyeong Cho, Won-Ja Lee
Veterinary Parasitology.2014; 205(3-4): 512. CrossRef - Prevalence and Genetic Characterization of Toxoplasma gondii in Bats in Myanmar
Hongchao Sun, Yiyin Wang, Yingguang Zhang, Wei Ge, Fuqiang Zhang, Biao He, Zuosheng Li, Quanshui Fan, Wei Wang, Changchun Tu, Jiping Li, Quan Liu
Applied and Environmental Microbiology.2013; 79(11): 3526. CrossRef