Abstract
The aim of the present study was to investigate antimalarial drug pressure resulting from the clinical use of different antimalarials in Thailand. The phenotypic diversity of the susceptibility profiles of antimalarials, i.e., chloroquine (CQ), quinine (QN), mefloquine (MQ), and artesunate (ARS) in Plasmodium falciparum isolates collected during the period from 1988 to 2003 were studied. P. falciparum isolates from infected patients were collected from the Thai-Cambodian border area at different time periods (1988-1989, 1991-1992, and 2003), during which 3 different patterns of drug use had been implemented: MQ + sulphadoxine (S) + pyrimethamine (P), MQ alone and MQ + ARS, respectively. The in vitro drug susceptibilities were investigated using a method based on the incorporation of [3H] hypoxanthine. A total of 50 isolates were tested for susceptibilities to CQ, QN, MQ, and ARS. Of these isolates, 19, 16, and 15 were adapted during the periods 1988-1989, 1991-1993, and 2003, respectively. P. falciparum isolates collected during the 3 periods were resistant to CQ. Sensitivities to MQ declined from 1988 to 2003. In contrast, the parasite was sensitive to QN, and similar sensitivity profile patterns were observed during the 3 time periods. There was a significantly positive but weak correlation between the IC50 values of CQ and QN, as well as between the IC50 values of QN and MQ. Drug pressure has impact on sensitivity of P. falciparum to MQ. A combination therapy of MQ and ARS is being applied to reduce the parasite resistance, and also increasing the efficacy of the drug.
-
Key words: Plasmodium falciparum, in vitro testing, Thailand, drug pressure
INTRODUCTION
Malaria continues to be a major public health problem in Southeast Asia, especially along the borders of Thailand. These regions have been historically linked with the emergence and spread of
Plasmodium falciparum parasites resistant to antimalarial drugs. Resistance to chloroquine, a 4-aminoquinoline, was first reported in Thailand in 1962, and has since spread rapidly throughout the country [
1]. There is a high prevalence of multidrug resistant falciparum malaria, and this includes resistance to structurally related antimalarial aminoquinolines such as quinine and mefloquine [
2]. One of the major reasons for the development and spread of drug resistance is selective drug pressure caused by antimalarial drug use. Different antimalarial drug exposure patterns in Thailand over the past decade has resulted in the natural selection of parasite genetic diversities, and thus parasite phenotype diversities and drug susceptibilities [
3].
Chloroquine was used clinically in Thailand in 1965-1974 and was followed by Fansidar®(sulphadoxine/pyrimethamine; 1974-1980), quinine (1980-1986), and mefloquine (1986-1995). Presently, a combination therapy incorporating mefloquine and artemisinin derivatives is used as a first-line treatment for uncomplicated falciparum malaria throughout the country (Ministry of Public Health of Thailand).
The aim of the present study was to investigate the influence of antimalarial drug pressure that has resulted from the clinical usage of different antimalarials (chloroquine, quinine, mefloquine, and artesunate) in Thailand and their phenotypic effects (susceptibility profiles) in P. falciparum isolates collected from 1988 to 2003. Results from this work may be of assistance in the rational design of antimalarial regimens for multidrug resistant P. falciparum.
MATERIALS AND METHODS
Culture system for parasite maintenance
P. falcifarum isolates were collected from endemic areas along the Thai-Cambodian border during the periods 1988-1989 (19 isolates from Chantaburi province), 1991-1993 (16 isolates from Trad provinces), and 2003 (15 isolates from Chantaburi province). All were kindly provided by Malaria Research Unit, Chulalongkorn University, Thailand. Isolates were maintained by culturing according to the methods of Trager and Jensen [
4], with modifications. Laboratory strains used in the study were the 3D7 (chloroquine-sensitive) and K1 (chloroquine-resistant) clones.
In vitro drug sensitivity assay
Sensitivities of
P. falciparum isolates to chloroquine (CQ), quinine (QN), mefloquine (MQ) and artesunate (ARS) were investigated based on the incorporation of [
3H] hypoxanthine into parasite nucleic acids [
5]. The method is based principally on measuring the rate of parasite growth via the incorporation of [
3H] hypoxanthine into nucleic acids. Highly synchronous ring stage parasites were used in each assay. The packed infected RBCs were diluted with fresh uninfected RBCs and complete medium to produce a final inoculum with 1% parasitemia and 20% hematocrit. Each assay was performed in triplicate. Microtiter plates (96-wells) were dosed with 8 different final concentrations of antimalarial drugs as follows: MQ (1, 5, 10, 25, 50, 100, 150, and 200 nM), CQ (5, 10, 25, 50, 100, 150, 250, and 500 nM), QN (10, 25, 50, 100, 150, 250, 500, and 1,000 nM), and ARS (0.1, 0.2, 0.4, 0.8, 1.0, 5.0, 7.5, and 10.0 nM). Data were represented graphically in the form of a log dose response curve plotted using the Grafit computer program (Erithacus Software Ltd., London, UK). The program was used to calculate drug IC
50 (drug concentration that inhibits the uptake of
3H-hypoxanthine by 50%) values via interpolation of the log dose response curve at the 50% growth marked on the ordinate axis. The IC
50 values were used as markers of drug potency, and permitted the direct comparison of the effects of the drugs used in the study.
The distribution of data was assessed using Kolmogorov-Smimov test. The in vitro activity of antimalarials was presented as the median of the IC50 values. Comparisons of median drug IC50 values for P. falciparum isolates collected during different time periods were performed using the Kruskal or Mann-Whitney U-test. The potential for in vitro cross-resistance was evaluated by the Spearman's correlation test. For all statistical tests, the significance level (P) was set at 0.05.
RESULTS
In vitro drug susceptibility of P. falciparum isolates
Fifty isolates were tested for their susceptibilities to CQ, QN, MQ, and ARS. Of these, 19, 16, and 15 isolates originated from the periods 1988-1989, 1991-1993, and 2003, respectively. The susceptibility levels of CQ, QN, and MQ were determined using criteria outlined by Cerutti et al. [
6] which discriminates between resistant and sensitive parasite isolates. However, there have been no criteria defining ARS resistance.
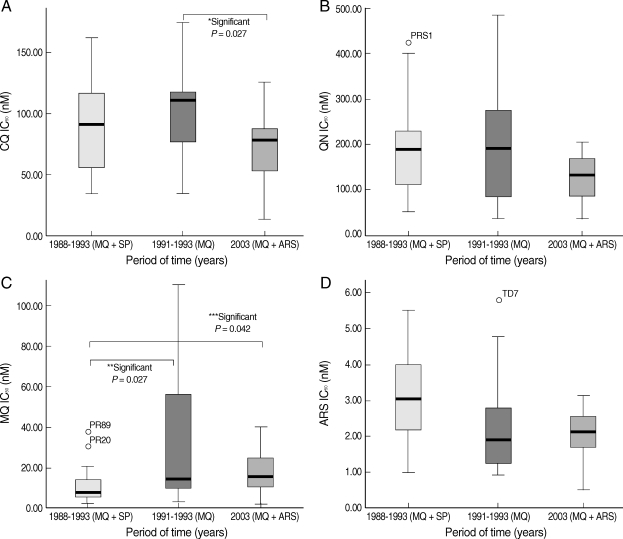
Chloroquine sensitivity
The median (95% confidence interval [CI]) of CQ IC
50 values for parasite isolates collected during 1988-1989, 1991-1993, and 2003 were 91 (51-119), 110 (67-119), and 78 (46-96) nM, respectively (
Fig. 1A). Median CQ IC
50 values of parasite isolates collected during 1991-1993 (period of clinical use of MQ monotherapy) were significantly higher than those collected during 2003 (period of clinical use of MQ and ARS combination) (
P = 0.027). The CQ IC
50 values of
P. falciparum isolates ranged from 13.8 to 174.2 nM. CQ susceptibilities were placed into 3 categories; sensitive (S : IC
50 < 25 nM), moderately resistant (MR; 25 ≤ IC
50 < 100 nM), and highly resistant (HR : IC
50 ≥ 100 nM). Based on these criteria, 1 (2%), 29 (58%), and 20 (40%) isolates were categorized as S, MR, and HR, respectively [
6]. Of the isolates collected during 1988-1989, 12 (63%) and 7 (37%) isolates were categorized as MR and HR, respectively; the corresponding prevalence for parasite isolates collected during 1991-1993 were 6 (37.5%) and 10 (62.5%), respectively. The prevalence of S, MR, and HR, for parasite isolates collected during 2003, was 1 (7%), 11 (73%), and 3 (20%), respectively.
QN susceptibility
The IC
50 values of QN for
P. falciparum isolates ranged from 34 to 483 nM. Using Pickard's criteria, all of the isolates were categorized as QN-sensitive isolates since their IC
50 values were less than 500 nM. The median (95% CI) IC
50 values for parasite isolates collected during 1988-1989, 1991-1993, and 2003 were 188 (99-231), 191 (66-281), and 129 (82-173), respectively. The median QN IC
50 values of isolates from the different time periods are displayed in
Fig. 1B. There were no significant differences in the IC
50 values among the isolates collected during the different time periods.
MQ susceptibility
The MQ IC
50 values of the
P. falciparum isolates ranged from 1.7 to 110 nM. The isolates were categorized as MQ-resistant or MQ-sensitive if their IC
50 values were > 24 nM and ≤ 24 nM, respectively. Based on these categories, 37 (74%) and 13 (26%) isolates were defined as MQ-sensitive and MQ-resistant, respectively. The median (95% CI) IC
50 values for parasite isolates collected during 1988-1989, 1991-1993, and 2003 were 7.86 (5.2-13.7), 14.4 (8.8-57.1), and 15.6 (10.2-25.1) nM, respectively (
Fig. 1C). The median MQ IC
50 values for parasite isolates collected during 1991-1993 and 2003 were significantly higher than those collected from 1988-1989 (
P = 0.027 and
P = 0.042, respectively).
ARS susceptibility
The ARS IC
50 values for
P. falciparum isolates ranged from 0.51 to 5.8 nM. The median (95% CI) IC
50 values for isolates collected during 1988-1989, 1991-1993, and 2003 were 3.05 (2-4.1), 1.9 (1.2-2.8), and 2.1 (1.6-2.8) nM, respectively. The median parasite isolate ARS IC
50 values during 1988-1989 was significantly higher than those collected during 1991-1993 (
P = 0.04) and 2003 (
P = 0.018) (
Fig. 1D).
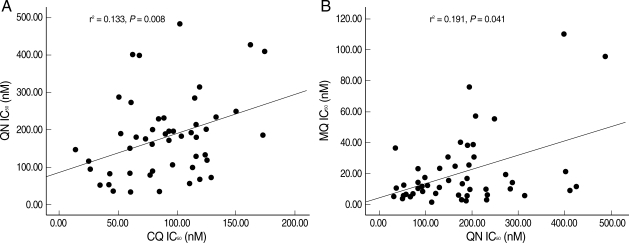
Correlations between the in vitro sensitivities of
P. falciparum isolates to CQ, QN, MQ, and ARS were determined using Spearman's correlation test (
Fig. 2A). A significantly positive but weak correlation between the IC
50 values of CQ and QN (
r2 = 0.133,
P = 0.008) and between the IC
50 values of QN and MQ (
r2 = 0.191,
P = 0.041) was observed (
Fig. 2B).
DISCUSSION
In our study,
P. falciparum isolates collected during the 3 periods demonstrated a wide variation in CQ IC
50 values, ranging from 13.8 to 174.2 nM. The median IC
50 value of the parasite isolates collected during 2003 was approximately 1.5 times lower than those collected from 1991-1993. Thus, our results have revealed a trend of increasing sensitivity to CQ, which is in keeping with the findings of previous reports [
7,
8]. This restoration of parasite CQ sensitivity may be due to the cessation of its clinical use in 1974. However, the sensitivity remains at resistant levels. Since CQ has been used as a first-line treatment for
Plasmodium vivax and
Plasmodium ovale, and due to the fact that these parasites share the same endemic areas with
P. falciparum, these isolates would be expected to have been exposed to pressure by CQ. This contrasts somewhat to the situation in Malawi where in vitro and in vivo experiments demonstrate
P. falciparum CQ sensitivity. Furthermore, they reveal a declining prevalence of CQ resistance conferring a mutation in the PfCRT at position T76 following the discontinuation of CQ clinical use in the country for the treatment of all malaria cases [
9,
10]. Moreover, a report on Cambodian isolates have shown a decline in CQ susceptibility (geometric IC
50 of CQ = 117 nM), indicating that CQ resistance in Cambodia is likely to remain stable and prevalent over time. This is despite the fact that CQ has been officially abandoned as a treatment regime for falciparum malaria in this country [
11].
All
P. falciparum isolates collected in the present study were found to be sensitive to QN (IC
50 values ranging from 34.5 to 483.4 nM). Similar patterns of sensitivity occurred during the 3 time periods for the different drugs. The lack of antimalarial drug resistance here might be explained by the limited clinical use of this drug, which had been restricted only to severe falciparum malaria cases, and additionally as a second-line treatment for acute uncomplicated falciparum malaria used in combination with tetracycline [
2]. The widespread use of QN in Thailand during the 1980s as a replacement therapy for SP resistance resulted in a significant reduction in QN sensitivity [
12-
14]. However, for the past 2 decades the drug has been used in combination with a partner antibiotic (tetracycline or doxycyline) to improve the clinical efficacy of QN, which might eventually result in the restoration of parasite QN sensitivity. The advantage of combination therapy is to achieve synergistic or additive antimalarial effect, and thus the parasite would be effectively cleared from the circulation without leaving the residuals to expose to subtherapeutic drug concentration [
16].
The in vitro sensitivity data showed that the majority of the isolates collected during the 3 study periods were considered sensitive to MQ (74%), with 26% in the resistant category. The prevalence of isolates with resistant phenotypes increased gradually from 16% during 1988-1989 to 47% in 2003. The sensitivities of the parasite isolates, as indicated by IC
50 values, also decreased (increased IC
50 values) from 1.6- to 3-fold from 1988 to 2003. However, significant differences were observed only when compared to the sensitivity data between the 1988-1989 and 1991-1993 time periods. MQ was implemented as the main antimalarial drug compound for treating uncomplicated falciparum malaria during the 3 observation periods, i.e., as MQ in combination with SP (1988-1989), MQ alone (1991-1993) and MQ in combination with ARS (2003), and thus continuous MQ drug pressure would be expected. In the present study, the sensitivity of the parasite to QN is higher than that reported previously but the pattern of declining MQ susceptibility is similar to those observed in regions of South East Asia [
7,
8,
11]. The in vitro sensitivity data we have documented here is also in keeping with the recent studies with MQ-ARS combination therapy used in the Trad province (an area along the Thai-Cambodian border) where clinical efficacy has dropped to 78.6% [
15]. This finding is of major concern since this combination regimen represents the most effective means of combating multidrug resistant falciparum malaria in Thailand.
Currently, ARS plays an important role in controlling multidrug resistant
P. falciparum malaria in Thailand. The development of ARS resistance is offset by the drug's short elimination half-life and ability to reduce gametocyte carriage rate [
16]. Our in vitro results revealed a moderate sensitivity of
P. falciparum isolates in Thailand during the study periods, as demonstrated by the IC
50 values which decreased by approximately 1.5-fold during the 1991-1993 and 2003 time periods as compared with 1988-1989. There has been no evidence of
P. falciparum clinical resistance to this group of drugs, and as a consequence its use in combination with effective antimalarials such as mefloquine is recommended in order to maintain their clinical efficacy. In the future,
P. falciparum sensitivity towards this group of drugs should be closely monitored.
A number of studies have provided evidence of cross-resistance among certain groups of quinoline-containing antimalarial drugs (e.g. QN, MQ, and halofantrine), while possessing an inverse relationship between these drugs and CQ-sensitivity [
17-
19]. The sensitivity to artemisinin derivatives has been found to be correlated with quinoline antimalarials. In our study, however, a positive correlation of the sensitivity of
P. falciparum isolates to CQ and QN was also observed, in addition to the expected strong correlation between MQ and QN. This correlation between CQ and QN sensitivities is in agreement with that reported previously by our group in isolates collected from 1998 to 2003 [
8]. The weak correlation between CQ and QN could be explained by the fact that both are aminoquinoline antimalarials which could share resistance mechanism. No correlation between ARS sensitivity and quinoline antimalarials was observed. The discrepancy of in vitro results reported from various studies may be a result of several factors, including the number of isolates studied, the origin of the isolates collected, and the history of antimalarial treatment in each study area.
ACKNOWLEDGEMENTS
The study was supported by Thammasat University. We are grateful to Malaria Research Unit, Chulalongkorn University, Thailand for parasites isolates. We thank Matthew J. Cheesman for editing the manuscript.
References
Fig. 1
Box-plot of the chloroquine (CQ) IC50 (A), quinine (QN) IC50 (B), mefloquine (MQ) IC50 (C), and artesunate (ARS) IC50 (D) values of P. falciparum isolates collected during different time periods.
MQ + SP = mefloquine plus sulfadoxine/pyrimethamine; MQ = mefloquine; MQ + ARS = mefloquine plus artesunate.

Fig. 2Scatter diagrams and regression lines representing the relationship between (A) IC50 (nM) values of chloroquine (CQ) and quninine (QN) (Spearman's correlation test); and (B) IC50 (nM) values of quninine (QN) and mefloquine (MQ) (Spearman's correlation test).