Abstract
Although the Korean isolate KI-1 of Toxoplasma gondii has been considered to be a virulent type I lineage because of its virulent clinical manifestations, its genotype is unclear. In the present study, genotyping of the KI-1 was performed by multilocus PCR-RFLP and microsatellite sequencing. For 9 genetic markers (c22-8, c29-2, L358, PK1, SAG2, SAG3, GRA6, BTUB, and Apico), the KI-1 and RH strains exhibited typical PCR-RFLP patterns identical to the type I strains. DNA sequencing of tandem repeats in 5 microsatellite markers (B17, B18, TUB2, W35, and TgM-A) of the KI-1 also revealed patterns characteristic of the type I. These results provide strong genetic evidence that KI-1 is a type I lineage of T. gondii.
-
Key words: Toxoplasma gondii, Korean isolate, genotype
Toxoplasma gondii is an obligate intracellular protozoan parasite that infects all warm-blooded vertebrates. About one-third of the human population is chronically infected with this parasite, and infection is primarily caused by the ingestion of under-cooked meat that contains viable
T. gondii cysts or oocysts shed from the feces of infected cats. Although human infection with
T. gondii is usually asymptomatic, cervical or occipital lymphadenopathy and ocular toxoplasmosis occurs in some patients. Congenital infection or reactivation in immunocompromised cases may lead to life-threatening encephalitis [
1].
The population structure of
T. gondii is an important issue to understand its variable disease manifestations and epidemiology, and for developing new strategies for treatment, vaccination, and diagnosis. It comprises 3 major clonal lineages based on genotypes, i.e., the type I (includes strains like RH which are highly virulent), type II (includes avirulent strains like PLK and Beverley), and type III (includes avirulent strains like VEG and CTG), which were identified by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis of 6 independent single-copy loci [
2]. The type I strains are associated with severe or atypical ocular toxoplasmosis in infected immunocompetent adults and are overrepresented in congenital infections [
3]. On the contrary, the type II strains account for most clinical toxoplasmosis cases in immunocompromised patients [
2,
3]. The type III strains are found in patients with ocular toxoplasmosis [
4]. Although genomic sequence differences between the 3 genotypes occur at a frequency of less than 1%, the virulence phenotypes in mice are strikingly different, with the type I strains uniformly lethal in outbred mice with LD
100 = 1; in contrast, the type II and III strains are significantly less virulent with LD
100 ≥ 10
3 [
5].
In the Republic of Korea, the seroprevalence of
T. gondii infection is reported to be about 7% [
6,
7], and several clinical cases have been documented [
8]. In spite of the accumulation of clinical toxoplasmosis cases in humans in Korea, it was only recently that
T. gondii tachyzoites were definitively isolated from the blood of an ocular patient and successfully maintained in the laboratory [
9]. The virulence and tissue culture characteristics of this unique Korean isolate, named the KI-1, resemble those of the type I strains. However, the genetic evidence for designating the KI-1 as a type I strain has been limited to the cDNA sequencing and RFLP analysis of
SAG1,
ROP1, and
GRA1 [
10]. In the present study, we employed a recently established high-resolution genotyping method to verify the typing of the KI-1 isolate. This method uses PCR-RFLP of multilocus genetic markers of
T. gondii [
11]. We also performed genotyping by DNA sequencing
T. gondii microsatellites [
12].
To isolate the genomic DNAs of the KI-1 and RH strains of
T. gondii, tachyzoites maintained in mouse skin fibroblast cells were harvested. Genomic DNA was extracted with the G-DEX™ IIc genomic DNA extraction kit (Intron Biotechnology, Suwon, Korea). The purified genomic DNA was quantified by spectrophotometry, and used immediately or stored at -20℃ until use. For multilocus PCR-RFLP, genetic markers for 9 chromosomal loci (c22-8, c29-2, L358,
PK1,
SAG2,
SAG3,
GRA6,
BTUB, and
Apico) were selected, and PCR primers were generated, as described previously [
11]. The target markers were amplified by PCR using ExTaq™ DNA polymerase (TaKaRa Bio, Shiga, Japan). The PCR was performed at 95℃ for 4 min, followed by 35 cycles of 94℃ for 30 sec, 56℃ (in the cases of c22-8, c29-2,
PK1,
GRA6, and Apico) or 59℃ (in the cases of L358,
SAG2,
SAG3, and
BTUB) for 1 min, and 72℃ for 2 min. The PCR products were purified with the QIAquick™ PCR purification kit (Qiagen, Valencia, California, USA). To reveal the RFLP pattern, purified PCR products were digested with restriction enzymes (New England Biolab, Ipswich, Massachusetts, USA) appropriate to the marker studied (for c22-8,
BsmA I and
MboII; for c29-2,
Hpy-CH4IV and
RsaI; for L358,
HaeIII and
NlaIII; for
PK1,
AvaI and
RsaI; for
SAG2,
HinfI and
TaqI; for
BTUB,
BsiEI and
TaqI; for
GRA6,
MseI; for
SAG3,
NciI; and for Apico,
AflII and
DdeI), according to the method described elsewhere [
11]. The enzyme-digested PCR products were then electrophoresed on a 2.5% agarose gel, stained with ethidium bromide, and visualized under UV-light.
To analyze the patterns of tandem repeats in microsatellites, PCR was performed with primers specific for the five microsatellite markers of
T. gondii,
B17,
B18,
TUB2,
W35, and
TgM-A, as described previously [
12]. The amplifications were carried out with an initial denaturation step of 95℃ for 4 min, followed by 35 cycles of denaturation at 94℃, annealing at 52℃ (for
TgM-A) or 54℃ (for
B17,
B18,
TUB2, and
W35), and extension at 72℃ for 30 sec. The PCR products were confirmed by 1.5% agarose gel electrophoresis, and purified as described above. The DNA sequences of the purified PCR products were determined (SolGent, Daejeon, Korea) and compared with various
T. gondii microsatellite sequences obtained from the GenBank.
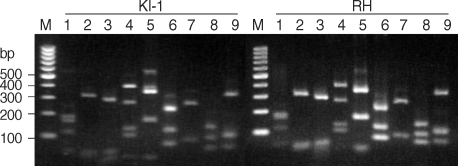
Although PCR-RFLP has been used to identify the genotypes of
T. gondii isolates, the genetic markers used have not always been representative of their phenotypes. For example, the frequent genetic crosses that occur in the parasite population diminish the association between the genotype and the phenotype. The Korean
T. gondii isolate designated as the KI-1 was identified as a type I with high virulence by PCR-RFLP with a set of conventional single-copy markers, i.e.,
GRA6 and
SAG2 [
10]. There is little information available on toxoplasmosis transmission rates and genetic crossing in relation to virulence in the Republic of Korea, therefore, we performed PCR-RFLP with multiple unlinked genetic markers and showed that this technique gave higher resolution [
11]. Su et al. [
11] developed 9 PCR-RFLP markers with each of them capable of distinguishing the 3 archetypal
T. gondii alleles in restriction-enzyme reaction by agarose gel electrophoresis. Through cluster analysis of 9 markers, each parasite can be discriminated the specific type from 3 major clonal lineages. All of the 9 amplified markers of c22-8, c29-2, L358,
PK1,
SAG2,
SAG3,
GRA6,
BTUB, and
Apico showed the expected molecular masses in the KI-1 and RH genomes (data not shown). The restricted enzyme-digested products were resolved by agarose gel electrophoresis (
Fig. 1). The marker resolutions for the KI-1 and RH strains were identical to the number and sizes of the bands identified for
T. gondii type I strain, which has previously been described as typical [
11]. Taken together with the results from PCR-RFLP with conventional markers [
10], the present results support the notion that the KI-1 belongs to the type I lineage.
PCR-RFLP of genetic markers has been shown to be effective in the identification of
T. gondii genotypes. However, there have been some technical problems, such as incomplete PCR amplification and restriction enzyme digestion [
15]. To overcome these problems, sequence analysis of tandem repeats in microsatellites was performed, as described previously [
13]. Tandem repeats of 5 amplified microsatellites,
B17,
TUB2,
W35,
TgM-A, and
B18, in the KI-1 and RH strains, were sequenced and compared with those of other
T. gondii strains within the 3 major genomic types (
Table 1). The sequences and numbers of the repeats in the KI-1 and RH microsatellites were identical to those in
T. gondii type I strain BK. Multiple alignments of the microsatellite sequences revealed 100% identity between the KI-1, RH, and BK strains (
Table 2).
Based on the results of the genotyping with high-resolution markers and microsatellites, we conclude that the unique Korean isolate of T. gondii, the KI-1, should be considered as a new geographical type I strain. Although there is little available information regarding the origin of the KI-1, minor polymorphisms in the sequences of SAG1, ROP1, and GRA8 from the KI-1 suggest genetic differences between the KI-1 and RH strains. The question as to whether the strain KI-1 is biologically identical to the strain RH remains to be resolved.
ACKNOWLEDGEMENTS
This study was financially supported by a research fund of the Chungnam National University in 2007.
References
Fig. 1Agarose gel electrophoresis of nine PCR-restriction fragment length polymorphism markers. The enzyme-digested PCR products were electrophoresed on a 2.5% agarose gel. KI-1, Korean isolate 1 of Toxoplasma gondii; RH, RH strain of T. gondii; M, 100 bp ladder molecular size marker. Lane 1, c22-8; lane 2, c29-2; lane 3, L358; lane 4, PK1; lane 5, SAG2; lane 6, BTUB; lane 7, GRA6; lane 8, SAG3; lane 9, Apico.

Table 1.Comparison of microsatellite sequences between Toxoplasma gondii strains
Table 1.
|
Toxoplasma strain |
Typea
|
Tandem repeats in microsatellite sequence (GenBank accession numbers)
|
Reference |
|
B17
|
B18
|
TgM-A
|
TUB2
|
W35
|
|
KI-1 |
I |
(TC)10
|
(CA)10
|
(TG)9
|
(CA)8
|
(TC)10(TG)2
|
This study |
|
|
(EF567114) |
(EF567115) |
(EF567116) |
(EF567117) |
(EF567118) |
|
|
RH |
I |
(TC)10
|
(CA)10
|
(TG)9
|
(CA)8
|
(TC)10(TG)2
|
This study |
|
|
(unreported) |
(unreported) |
(unreported) |
(unreported) |
(unreported) |
|
|
BK |
I |
(TC)10
|
(CA)10
|
(TG)9
|
(CA)8
|
(TC)10(TG)2
|
Ajzenberg et al. (2004) |
|
|
(AY572751) |
(AY572714) |
(AY572650) |
(AY572593) |
(AY572621) |
|
|
ME49 |
II |
(TC)7
|
(CA)9
|
(TG)8
|
(CA)7
|
(TC)7(TG)2
|
Ajzenberg et al. (2004) |
|
|
(AY572759) |
(AY572710) |
(AY572661) |
(AY572581) |
(AY572631) |
|
|
NED |
III |
(TC)7
|
(CA)10
|
(TG)7
|
(CA)7
|
(TC)6(TG)3
|
Ajzenberg et al. (2004) |
|
|
(AY572760) |
(AY572726) |
(AY572662) |
(AY572595) |
(AY572632) |
|
Table 2.Sequence of Toxoplasma gondii microsatellites of KI-1, RH, and BK strains
Table 2.
|
Microsatellite marker |
Sequences |
Size (bp) |
|
B17 |
GCCGCCTTCC |
TCTTCCACTT |
TTCTTCCTCT |
CTTTGCCCCT |
TAGTGGCCTC |
200 |
|
|
GACTCTTGCT |
GTACTCCGCT |
TCCCTGTGTG |
TCACCGTTCC |
TTCCTTCTTC |
|
|
|
TATCTTCCCT |
TCTCTCTCTC |
TCTCAGAGAA |
CTCCCTGCGT |
TCCATCCCCT |
|
|
|
CTCCCCTTCT |
TCAGTTGTTC |
TCTTGCTCTT |
GTGTCTCTCC |
TCCTCTACCT |
|
|
B18 |
AACAGAGGTT |
TTTCTTGGTG |
TGCGGAATCC |
CACACACACA |
CACACACACA |
105 |
|
|
GCTACAGGCA |
TTGCCATCAG |
TGGGGGTTCT |
GCGCCATTAT |
CTTTTGGATG |
|
|
|
AAAGG |
|
|
|
|
|
|
TgM-A |
TGCATGCATG |
TCCCTGTCGG |
TTTCTCCGTG |
TTTCCATGTG |
TGTGTGTGTG |
149 |
|
|
GTGTTTGTAA |
GTCGAAGAAG |
TTCCGATTTA |
TGCATCTGCA |
CGTGGACTT |
|
|
|
TTTTATGTGG |
TAGTTTCCAT |
CCGCATTTAC |
GGAGCTCTAT |
TTACATGCC |
|
|
TUB2 |
TGGCCAAACG |
GACCCGCGCG |
AACGCTGTCC |
ATAGTGCCCG |
GCTCCAGATC |
258 |
|
|
CATGAGGATC |
GCACGCGGGA |
CGAAGCGTCC |
ACCGGTGGCC |
TCATTGTAGA |
|
|
|
ACACATTGAT |
TCTCTCCAGC |
TGCAAGTCAC |
TGTCTCCACA |
GTAGGTACCG |
|
|
|
GTCTGAAGAG |
AGGAAACCAC |
ACACACACAC |
ACATCGCAGA |
ACAAATTGAA |
|
|
|
ATGACGGAAG |
AACTTGGCCG |
ATGTCGCCGC |
GCCTGCATTT |
TGTAGGAACA |
|
|
|
CCCGGACA |
|
|
|
|
|
|
W35 |
CGTCGCCCGT |
CCTGCTGCGG |
TCTTTTCTCT |
TCTTCTTGGC |
TTTCTCTCTC |
212 |
|
|
TCTCTCTCTC |
TCTGTGTCGC |
TGTGCGGATA |
AAACAGCGCG |
CGTCTTTGTG |
|
|
|
GGAAGGGAAC |
GCATGTTCC |
CTTCGTTTAT |
GTTCATCTGC |
ACTCTCTTCG |
|
|
|
CATTTGCGAA |
CTCGAGGTTT |
CCAGATATC |
TCGGAGTCCG |
CTGTCGGAAA |
|
|
|
CAAGCGACGT |
TC |
|
|
|
|