Abstract
Serum from mouse orally ingested with tissue cyst forming strain (Me49) of Toxoplasma gondii was assayed by Western blot and immunofluorescene assay (IFA) to establish early responses in antigenicity of the parasite in mouse model of foodborne toxoplasmosis. Sera were collected weekly to blot the RH antigen transferred onto nitrocellulose paper after being separated by 12% SDS-PAGE. With the second week serum, 34 kDa protein (p34) was detected uniquely, and all antigens of T. gondii were detected with the sera from 3 or 4 weeks. p34 was not a member of the major surface membrane proteins and confirmed to be localized in the rhoptry by IFA. It was secreted into parasitophorous vacuolar membrane (PVM) during the entry into host cells. When applied to the human sera of which the ELISA absorbance was in negative range, 10.3% of sera detected p34, while all the ELISA positive sera detected the band. It has diagnostic usefulness of presumed T. gondii infection. We suggest the name of the p34 protein as ROP9.
-
Key words: Toxoplasma gondii, tissue cyst forming strain, peroral ingestion, p34, rhoptry protein, ROP9, PVM secretion, diagnostic availability
INTRODUCTION
Toxoplasma gondii is an obligate intracellular parasite which can infect many warm-blooded animals including humans. It is the causative agent of congenital toxoplasmosis and severe opportunistic infections in immunocompromised patients (
Luft and Remington, 1992;
Remington et al., 1995).
Postnatal infection of
T. gondii is induced mainly by ingesting food and water contaminated with oocysts passed in feces of infected cats or by ingesting tissue cysts in raw/undercooked infected meat (
Dubey, 1988). Toxoplasmosis is generally asympto-matic in healthy persons, but, recently we have reported symptomatic chorioretinitis or lymphadenitis outbreaks regardless of underlying status of health after ingestion of tissue cysts in raw/undercooked pork (
Choi et al., 1997). Since McLeod et al. (
1984) tried mouse model of peroral ingestion to define the immune responses of host, peroral route of infection has been used widely to analyse the events that might occur in the early stage of infection (
Liesenfeld et al., 1997;
Jebbari et al., 1998;
Lee et al., 1999).
Until now the antibody reaction of the host especially to detect early infection has not been established. Therefore, we have tried Western blot and immunofluorescence assay (IFA) with the sera collected from mice orally ingested with tissue cyst forming strain of T. gondii.
MATERIALS AND METHODS
Antigen preparation
RH tachyzoites of
T. gondii were maintained by a peritoneal passage in inbreeding mice (ICR strain) and purified by centrifugation over 40% Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden) in PBS solution (
Choi et al., 1992).
Me49 strain, a tissue cyst forming strain of T. gondii, was used to ingest the mice orally. Infected brain of mice was homogenized with a mortar and pestle, and then the number of cysts were counted under a microscopy. One hundred cysts were fed per mouse of 4-week-old Balb/c strain. Eight sera were collected per each group at 1, 2, 3, 4 and 8 weeks after ingestion and freezed at -70℃ until use.
For the diagnostic applicability of p34 to presumed early infection in human, a total of 54 anti-
T. gondii positive sera of absorbance at 490 nm equal to 0.25 or higher in ELISA (
Choi et al., 1992) and a total of 126 anti-
T. gondii negative sera of absorbance below 0.25 were tested to count the number of reactive sera of each group with p34.
Western blot was done by the method of Towbin et al. (
1979). Whole extracts of tachyzoites (RH strain) were separated in a 12% SDS-PAGE gel and transferred onto nitocellulose (NC) papers (Schlleicher and Shuell, Keene, NH, USA). NC papers were incubated with sera of 1:100 dilution and then with 1:2,000 diluted horse redish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (Cappel, Costa Mesa, CA, USA). They were soaked in enhanced chemiluminacence (ECL) solution (Amersham Pharmacia Biotech) for 1 min and exposed to X-ray film (Konica, Tokyo, Japan) for 5 to 10 sec. For the detection of the surface membrane proteins, RH tachyzoites were labelled with sulfo-NHS-biotin (Pierce, Rockford, IL, USA) and blotted with avidin-HRP (Pierce) by the method of Cole et al. (
1987). Biotin-avidin binding was developed with 4-chloro-1-naphthol (Sigma, St. Louis, MO, USA) in the presence of 0.01% H
2O
2.
For the immunofluorescence assay (IFA), freshly prepared RH tachyzoites suspended in phosphate buffered saline (PBS) were cytospinned onto 18-mm coverslips. And NIH 3T3 cells cultured on coverslips were infected with RH tachyzoites for 2 and 24 hr. Cells were fixed with cold absolute methanol for 5 min or fixed with paraformaldehyde for 10 min and then permeabilized the membraneous structure with Triton X-100 for 5 min, separately. Sera were applied with 1:100 dilution in incubation solution (3% bovine serum albumin in PBS) and coverslips were incubated with 1:500 diluted FITC-conjugated goat anti-mouse IgG antibody (Sigma). Fluorescence was observed under a fluorescence microscopy (Axiophot, Carl Zeiss, Oberkochen, Germany).
RESULTS
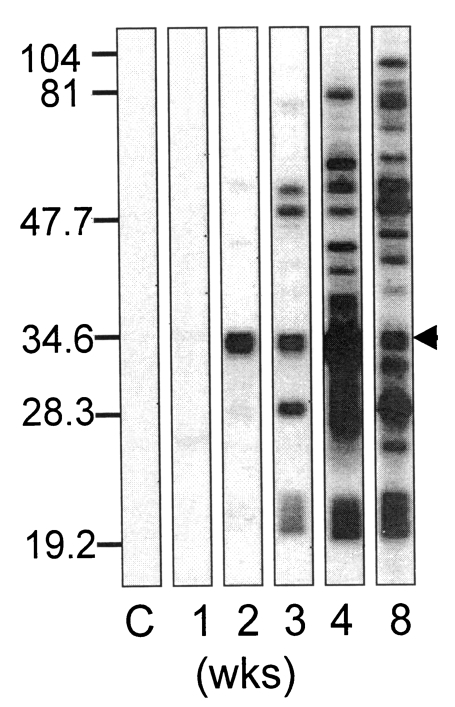
Sera of mice ingested with Me49 strain of
T. gondii reacted with whole extract antigens from 2 weeks after ingestion as shown in
Fig. 1. With the sera of 2 weeks, only one major band reacted distinctly, and maintained reactivity until the end of experimental period of 8 weeks (indicated by arrow), of which the molecular weight was estimated as 34 kDa (p34). At this time point, bradyzoite or small sized cysts were found in the brain of mice (data not shown) as described well previously (
Dubey, 1997). With the sera of 3 weeks, bands over 50 kDa and those of 28 kDa and 22/23 kDa were detected in addition to the 34 kDa band. And from 4 weeks after ingestion almost all antigens of crude extract were detected.
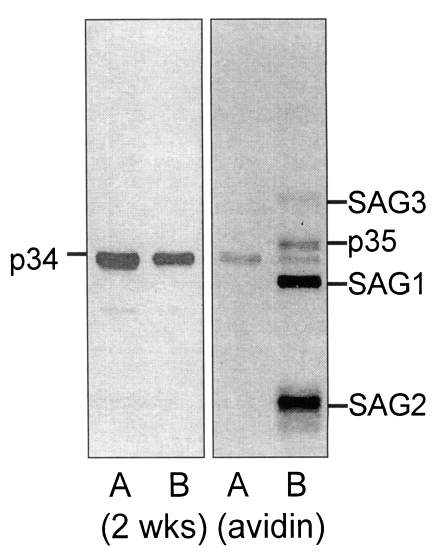
The p34 antigen detected first as early as 2 weeks after ingestion was not equivalent to the one of the major surface proteins, p35, as shown in
Fig. 2. Wild (not biotinylated) RH extract and surface biotinylated RH extract were separated to blot with the 2 weeks serum, and then with avidin-HRP continuously. Two weeks serum detected p34 in both wild and biotinylated RH extracts (
Fig 2, left A, B). When avidin-HRP was applied to the same NC paper, four major surface proteins of
T. gondii, 43 kDa (SAG3), p35, 30 kDa (SAG1), and 22 kDa (SAG2) (
Couvreur et al., 1988) were detected only in biotinylated RH extracts (
Fig. 2, B of the right panel) in addition to the vestigial bands of p34 in both lanes.
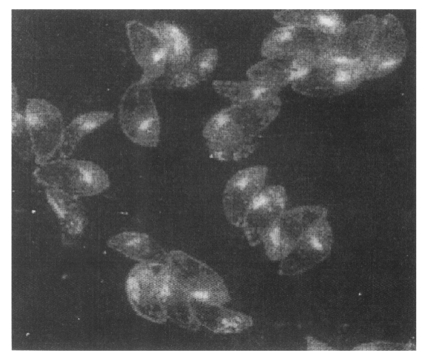
By IFA with the 2 weeks serum on the extracellular RH tachyzoites, club-shaped apical portion was stained as shown in
Fig. 3, which might be comparable with the rhoptry organelle as depicted in electron microscopy by Dubremetz (
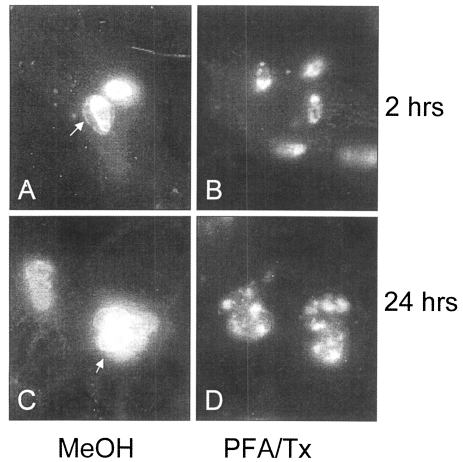
1998). And when applied to the intracellular tachyzoites in NIH 3T3 cells at 2 and 24 hr after infection, p34 was secreted into parasitophorous vacuolar membrane (PVM) as in
Fig. 4A and 4C (arrows), which disappeared when permeabilized by Triton X-100 (
Fig. 4 B, 4D).
Focused on the early detectability of p34, preliminary trial of p34 availability was performed to detect presumed early infections that were diagnosed as negative when the infections are too early to reach the enough antibody titers. Among 126 anti-T. gondii negative sera of human, 13 sera (10.3%) detected p34 band while all 54 anti-T. gondii positive sera detected p34.
DISCUSSION
Antibody formation after ingestion of tissue cyst forming strain of
T. gondii into mouse was traced to establish the profile of the antigenicity of
T. gondii proteins as an animal model of food-borne toxoplasmosis. Regardless of
T. gondii-related symptoms after ingestion, murine sera collected weekly revealed typical antibody patterns among individuals at the same week. Especially at the second week, 34 kDa antigen was detected uniquely and was maintained for the entire experimental period. Detection of p34 antigen occurred prior to that of p30 (SAG1,
Lunden et al., 1993;
Yano et al., 1997) or p22 (SAG2,
Parmley et al., 1992) which had been reported to be strong antigenic but detected from the third week as shown in
Fig 1. The missing to detect p34 from infected animals may be resulted from the different time points to collect the sera after infection or the use of less sensitive detection methods.
By double immunoblotting with 2 weeks serum and avidin on the same NC paper, it was defined clearly that the early detected antigen was not overlapped with p35, which may be misunderstood as a member of four major surface membrane proteins of
T. gondii when performed separately. The blotting procedure should be ideal when avidin first and then 2 weeks serum later, but the procedure was reversed because ECL image with avidin-HRP was too much sensitive to differentiate two bands clearly (data not shown). Therefore, color development with 4-chloro-1-naphthol was chosen instead of ECL, and the substrate was precipitated in the bands of four major surface proteins with vestigial bands of p34 as in
Fig. 2.
By IFA, p34 was confirmed to be located in rhoptry organelle and was secreted into PVM as early as 2 hr after infection of host cells. Rhoptries are the peculiar structural feature of all the invasive forms of apicomplexan parasites and are implicated in playing a central role in host cell invasion (
Perkins, 1992). And rhoptry proteins are named as follows; 68/60.5 kDa ROP1, 66/55 kDa ROP2, 66/59 kDa ROP3, 60 kDa ROP4, 59.5 kDa ROP5, 42 kDa ROP6, 56 kDa ROP7, and 52 kDa ROP8, respectively (
Sam-Yellowe, 1996). Although the function of p34 and genetic data are not available until now, we suggest the name of p34 as ROP9 after a recent identification of the 52 kDa protein to be located at the rhoptry organelle, ROP8 (
Beckers et al., 1997).
Recently, PCR has been used to detect congenital infection or reactivation of immunocompromised patients such as AIDS (
Hohlfeld et al., 1994;
Gratzl et al., 1998), indirect latex agglutination test or ELISA has been used generally in diagnosis and epidemiologic screening (
Choi et al., 1992;
Bhopale et al., 1997;
Jenum et al., 1997). But in the latter it has space to fail in diagnosis of early infection or ingestion, when the antibody titers are too low to be estimated as positive. p34 is recommended as a candidate antigen to be used in those purposes strongly. Actually, p34 was blotted with 10.3% human sera of negative ELISA absorbance and with 100% positive sera, which suggests the diagnostic availability of this antigen for the early infection/ingestion of
T. gondii as well as established toxoplasmosis.
Notes
-
This study was supported in part by a grant from The Korea Science and Engineering Foundation (KOSEF-961-0716-101-1).
References
- 1. Beckers CJ, Wakefield T, Joiner KA. The expression of Toxoplasma proteins in Neospora caninum and the identification of a gene encoding a novel rhoptry protein. Mol Biochem Parasitol 1997;89:209-223.
- 2. Bhopale GM, Naik SR, Bhave GG, Naik SS, Gogate A. Assessment of enzyme linked immunosorbent assay based diagnostic kits (Toxokit-G and Toxokit-M) for the detection of IgG and IgM antibodies to Toxoplasma gondii in human serum. Comp Immunol Microbiol Infect Dis 1997;20:309-314.
- 3. Choi WY, Nam HW, Kwak NH, et al. Foodborne outbreaks of human toxoplasmosis. J Infect Dis 1997;175:1280-1282.
- 4. Choi WY, Nam HW, Youn JH, et al. Detection of antibodies in serum and cerebrospinal fluid to Toxoplasma gondii by indirect latex agglutination test and enzyme-linked immunosorbent assay. Korean J Parasitol 1992;30:83-90.
- 5. Cole SR, Ashman LK, Ev PL. Biotinylation: an alternative to radioiodination for the identification of cell surface antigens in immunoprecipitates. Mol Immunol 1987;24:699-705.
- 6. Couvreur G, Sadak A, Fortier B, Dubremetz JF. Surface antigens of Toxoplasma gondii. Parasitology 1988;97:1-10.
- 7. Dubey JP. Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii. J Euk Microbiol 1997;44:592-602.
- 8. Dubey JP, Beattie CP. Toxoplasmosis of animals and man. 1988, Boca Raton, Florida, USA. CRC Press.
- 9. Dubremetz JF. Host cell invasion by Toxoplasma gondii. Trends Microbiol 1998;6:27-30.
- 10. Gratzl R, Hayde M, Kohlhauser C, et al. Follow-up of infants with congenital toxoplasmosis detected by polymerase chain reaction analysis of amniotic fluid. Eur J Clin Microbiol Infect Dis 1998;17:853-858.
- 11. Hohlfeld P, Daffos F, Costa JM, Thulliez P, Forestier F, Vidaud M. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N Engl J Med 1994;331:695-699.
- 12. Jebbari H, Roberts CW, Ferguson DJ, Bluethmann H, Alexander J. A protective role for IL-6 during early infection with Toxoplasma gondii. Parasite Immunol 1998;20:231-239.
- 13. Jenum PA, Stray-Pedersen B, Gundersen AG. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J Clin Microbiol 1997;35:1972-1977.
- 14. Lee YH, Channon JY, Matsuura T, Schwartzman JD, Shin DW, Kasper LH. Functional and quantitative analysis of splenic T cell immune responses following oral Toxoplasma gondii infection in mice. Exp Parasitol 1999;91:212-221.
- 15. Liesenfeld O, Kosek JC, Suzuki Y. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect Immun 1997;65:4682-4689.
- 16. Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis 1992;15:211-222.
- 17. Lunden A, Lovgren K, Uggla A, Araujo FG. Immune responses and resistance to Toxoplasma gondii in mice immunized with antigens of the parasite incorporated into immunostimulating complexes. Infect Immun 1993;61:2639-2643.
- 18. McLeod R, Estes RG, Mack DG, Cohen H. Immune response of mice to ingested Toxoplasma gondii: a model of Toxoplasma infection acquired by ingestion. J Infect Dis 1984;149:234-244.
- 19. Parmley SF, Sgarlato GD, Mark J, Prince JB, Remington JS. Expression, characterization, and serologic reactivity of recombinant surface antigen p22 of Toxoplasma gondii. J Clin Microbiol 1992;30:1127-1133.
- 20. Perkins ME. Rhoptry organelles of apicomplexan parasites. Parasitol Today 1992;8:28-32.
- 21. Remington JS, McLeod R, Desmonts G. In Remington JS, Klein JO eds, Toxoplasmosis. Infectious diseases of the fetus and newborn infant. 1995, Philadelphia, USA. WB Saunders; pp 140-167.
- 22. Sam-Yellowe TY. Rhoptry organelles of the Apicomplexa: their role in host cell invasion and intracellular survival. Parasitol Today 1996;12:308-316.
- 23. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 1979;76:4350-4354.
- 24. Yano A, Aosai F, Yang TH, et al. Correlation between direct binding ability of synthetic Toxoplasma gondii SAG1 peptides to HLA-A2 measured by a sensor for surface plasmon resonance and antigenicity of the peptides for Toxoplasma gondii-infected cell-specific CTL. Biochem Biophys Res Comm 1997;236:257-261.
Fig. 1Western blot of Toxoplasma gondii antigen with mouse serum collected at 1, 2, 3, 4, and 8 weeks after ingestion with Me49 strain of T. gondii. C indicates serum of uninfected mouse. Numerals of the left column indicates molecular weight of kDa. Arrow head indicates peculiar band which reacted uniquely with 2 weeks serum.
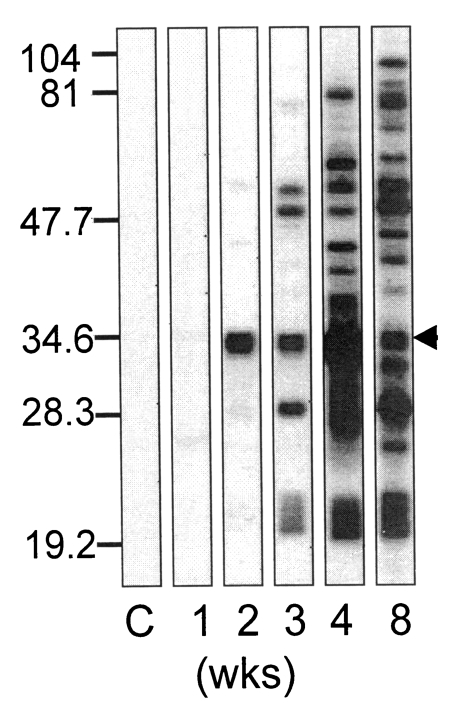
Fig. 2Western blot of Toxoplasma gondii antigen of wild (not-biotinylated) RH (A) and surface biotinylated RH (B) with 2 weeks serum (left panel), and then with avidin-HRP (right panel) on the same NC paper. Major biotinylated surface membrane proteins were designated as SAG3 (43 kDa), p35, SAG1 (30 kDa) and SAG2 (22 kDa), respectively.
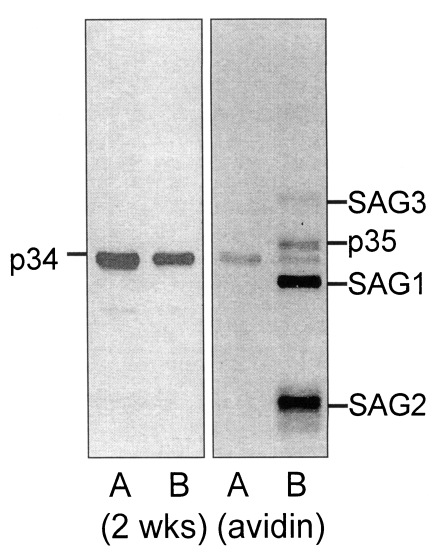
Fig. 3An IFA image with 2 weeks serum on the extracellular Toxoplasma gondii. Club-shaped apical portion was stained distinctly to be rhoptry organelle (×1,000).
Fig. 4IFA images with 2 weeks serum on the intracellular Toxoplasma gondii at 2 and 24 hr after infection of host cells fixed with methanol (MeOH) and permeabilized with Triton X-100 (PFA/Tx). Arrows indicates the secretion of p34 into PVM (×1,000).