Abstract
This study aimed to investigate the infection status and species diversity of trematode cercariae in freshwater snails from canal networks in the Bangkok Metropolitan Region (BMR), Thailand. The snails were collected from 35 sites during 2 cycles of the wet (July–October) and dry (November–June) seasons in 2018–2019. A total of 29,420 snails representing 24 species/subspecies were examined for cercarial infection using shedding and crushing techniques. We found that 1,275 snails from 12 species/subspecies were infected, resulting in an overall prevalence of 4.3%. Infections were significantly higher (P<0.001) during the wet season (5.9%; 970/16,473) than during the dry season (2.4%; 305/12,947). Morphological identification revealed 37 distinct types within 15 morphotypes, with the armatae morphotype showing the highest infection rate (1.8%) and the greatest cercarial diversity (8 distinct types). This study highlights the health risk posed by the Bithynia siamensis siamensis, which was the second most prevalent (8.5%) and hosted the greatest cercarial diversity (11 morphotypes, 15 distinct types). This subspecies also displayed a wide distribution range (31 localities) with a generally high occurrence frequency in the BMR. This study firstly documents a gymnophallid digenean as a freshwater digenean, presenting evidence of a dichotoma cercarial morphotype from 4 species/subspecies (Filopaludina martensi martensi, F. sumatrensis polygramma, B. siamensis siamensis, and Wattebledia siamensis) with a low infection range (0.1–0.4%). A staggering diversity of cercariae was observed in the BMR canal networks with seasonal fluctuations. The B. siamensis siamensis displayed notable epidemiological importance in the BMR flowing-water networks. This study provides quantitative and qualitative morphological descriptions and measurement guidelines for the dichotoma cercaria in Thailand.
-
Key words: Snail-transmitted parasite, gymnophallidae, dichotoma cercaria, freshwater habitat, seasonal variation
Introduction
Digenetic trematodes (Trematoda: Digenea) form a large and diverse group of endoparasitic flatworms, commonly known as flukes, that generally parasitize various vertebrates during the adult stage [
1]. Several digenean species have significant socio-economic and public health impacts, causing pathogenesis, bacterial invasion, inflammation, and severe morbidities and mortalities in fishes, livestock, and humans [
1–
7]. For instance, co-infections of Cyathocotylidae and motile aeromonads have been linked to the massive mortality of farmed Nile tilapia (
Oreochromis niloticus) [
5]. The ruminant blood flukes
Schistosoma spindale and
S. indicum (Schistosomatidae), which are widespread across South Asia, including Thailand, can cause visceral schistosomiasis, resulting in reduced milk yield and increased mortality in sheep and cattle [
7]. Additionally, an outbreak of cercarial dermatitis caused by ruminant and avian schistosomes has been reported among rice farmers in South Thailand [
6]. Notably, infections of the liver fluke
Opisthorchis viverrini sensu lato in humans, which can cause and induce severe complications and mortalities due to bile duct cancer, have been well-documented in the Mekong River countries, i.e., Thailand (primarily the northeastern region), Laos, Myanmar, Cambodia, and Vietnam [
8].
The life cycles of digenetic trematodes are complicated, typically involving at least 2 hosts [
1]. Vertebrates usually serve as definitive hosts where sexual reproduction occurs, while invertebrates, especially snails, act as intermediate hosts where asexual reproduction occurs. Briefly, digenean eggs are released into aquatic environments via the excreta of the definitive hosts. These eggs are either ingested by snail hosts or hatch into miracidia, which penetrate snail tissues and transform into sporocysts, within which several embryos develop asexually into rediae. Subsequently, the rediae produce numerous cercariae as the final product of the asexual phase. The cercariae then escape the snail hosts, swim to find compatible second intermediate hosts, and encyst as metacercariae. The metacercariae mature into adults after the ingestion of these second intermediate hosts by definitive hosts. Asexual reproduction within snail hosts possibly amplifies cercarial transmission to subsequent hosts during their life cycles, highlighting the vital role of snails in ecosystem dynamics. Furthermore, prevalence data of cercarial infections in snails serve as a valuable indicator of digenetic trematode transmissions from definitive hosts into ecosystems and is useful for predicting the epidemiological status of the targeted parasites [
8,
9].
In Thailand, investigations on cercarial infections in 1–20 freshwater snail species/subspecies encompassing 1–10 families have been conducted in various scales of administrative areas [
6,
9–
15]. The studies reported 6 to 19 morphologically distinct types across 4 to 9 morphotypes belonging to approximately 19 digenean families [
6,
9–
14,
16]. A study by Anucherngchai et al. [
9], which focused on central Thailand, reported 9 cercarial morphotypes belonging to 5 digenean families in 8 out of 14 examined freshwater snail species/subspecies in the Chao Phraya Basin. Chontananarth et al. [
11] also reported 7 cercarial morphotypes belonging to 7 digenean families in 14 freshwater snail species/subspecies from the Nakhon Nayok Province. The species
Melanoides tuberculata (Thiaridae) was identified as the most susceptible to cercarial infection. However, Wiroonpan et al. [
14], who comprehensively investigated cercarial infections in freshwater snails in Bangkok Metropolis, reported 8 cercarial morphotypes belonging to 10 digenean families in 12 infected snail species/subspecies; the
Bithynia siamensis siamensis (Bithyniidae) was the most susceptible to cercarial infection.
The Bangkok Metropolitan Region (BMR) is a flat alluvial plain in central Thailand that comprises the Bangkok Metropolis, Nonthaburi, Nakhon Pathom, Samut Sakhon, Samut Prakan, and Pathum Thani Provinces. Despite its modest size compared to the rest of the country, BMR is epidemiologically significant because it is the most densely populated area in Thailand with a substantial migrant workforce [
17]. In addition, this region features a vast array of water resources, including an extensive canal network catering to diverse land uses, such as agriculture, fisheries, livestock husbandry, and residential areas [
18,
19]. The BMR canal networks also presumably offer a diverse range of foods, habitats, and routes for the existence and distribution of digeneans and their hosts [
20,
21]. Moreover, most areas of the BMR canal networks are a public source of water where the country’s population can engage in activities such as swimming and fishing.
Despite the epidemiological significance of cercarial infections in molluscan intermediate hosts, there is a lack of comprehensive studies on trematode cercariae infections in freshwater snails from BMR. Therefore, this study aimed to investigate the infection status and species diversity of cercariae in freshwater snails from canal networks in the BMR in Thailand.
Materials and Methods
Ethics statement
Approval for this study (collecting, temporarily nurturing, and investigating trematode cercarial infections in freshwater snails) was obtained from the Kasetsart University Ethics Committee (Approval No. ACKU61-SCI-034).
Collection and nurture of snails
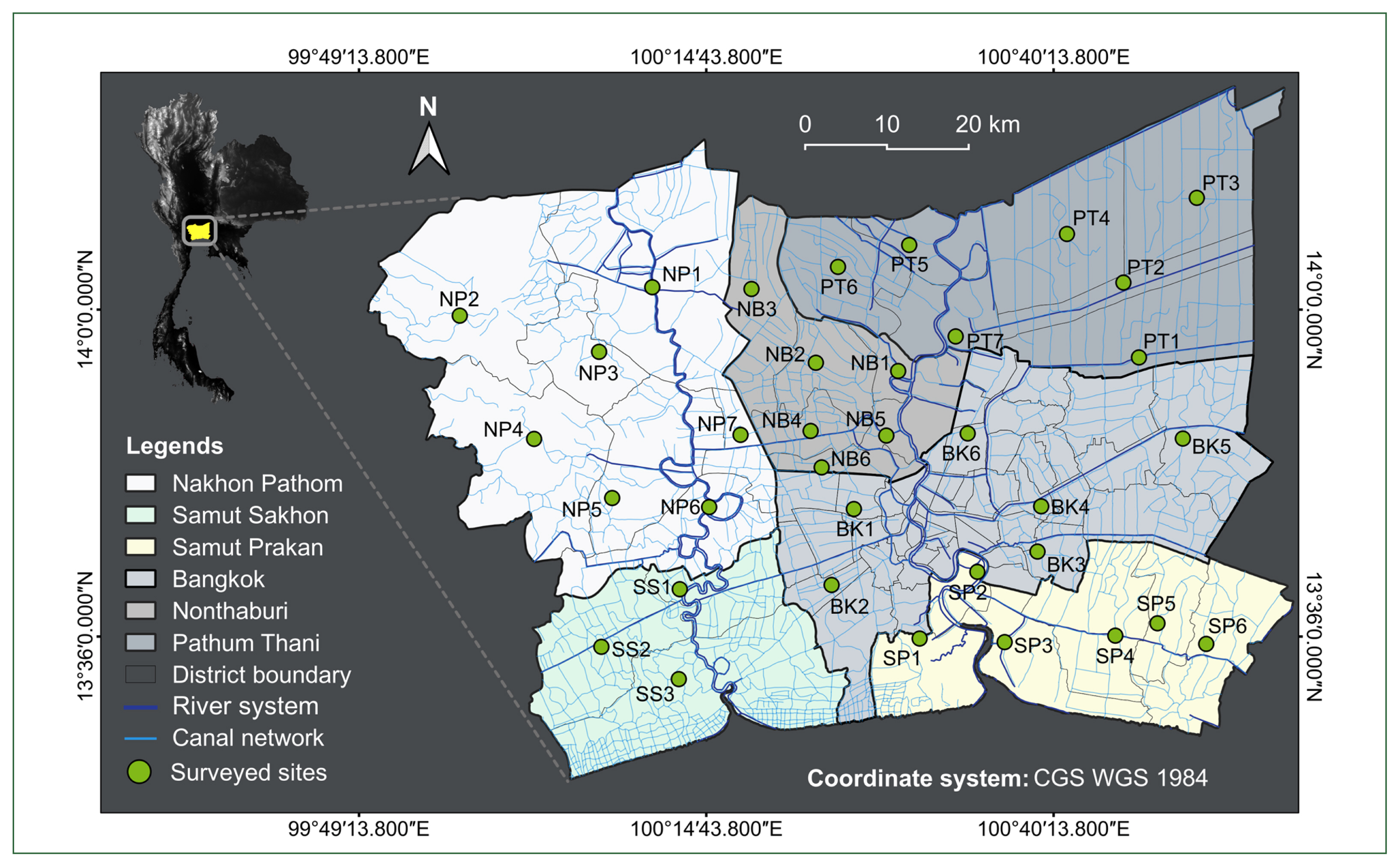
Thirty-five sites of the canal network encompassing all administrative zones/areas in the BMR (
Fig. 1;
Table 1) were selected for the survey. Random samplings of freshwater snails, carried out through hand-picking and scooping with a conventional wire-mesh scoop and a hand net, were executed by a single collector, allocating 20 min for each site. This approach was based on the count per unit of time sampling method [
22]. The samplings were conducted every 3 months from January 2018 through October 2019, covering 2 cycles of the wet (July–October) and dry (November–June) seasons. The collected snails were transported to the laboratory and identified based on the shell and operculum morphology according to taxonomic keys [
23,
24]. The snails were temporarily nurtured in 2.5-L perforated, transparent plastic boxes containing 2 L of dechlorinated water, with an appropriate snail-to-box ratio maintained throughout the investigation period for cercarial infection.
After acclimatization under laboratory conditions for one day, individual snail specimens were placed in 2-oz, 5-oz, or 9-oz transparent plastic cups, each filled to 3-quarters of its capacity with dechlorinated water (sized appropriately for the snails). Each cup was exposed to a light intensity of approximately 3,000 lux from 6:00 to 14:00 o’clock at room temperature (25±2°C) and then observed under a stereomicroscope. This shedding technique was performed on alternate days over 1 week. Subsequently, the crushing technique was employed, wherein the snail body was separated from its shell and operculum and crushed using Petri dishes containing a small volume of dechlorinated water; the samples were then examined under a stereomicroscope.
Morphological identification of the cercariae
The live cercariae detected in each shedding cup and those separated from the crushed snail tissues were transferred onto glass slides using glass Pasteur pipettes and fixed in a relaxed state using 10% neutral buffered formalin. Some cercarial specimens were vitally stained with 0.5% neutral red solution. The specimens were observed and photographed using an Olympus BX51 microscope equipped with an Olympus DP70 camera (Olympus Corporation, Tokyo, Japan). Multiplanar photomicrographs of mature cercariae were employed to identify them based on their morphological traits and movement behaviors according to the identification keys [
1] and descriptions [
6,
12–
14,
25]. The morphological features were illustrated using Affinity Designer v1.10.6 (Serif Ltd., Nottingham, UK) and the metric traits (only displayed for dichotoma cercaria) were measured using ImageJ v1.53t (National Institutes of Health, Bethesda, MD, USA) based on the guidelines shown in
Supplementary Fig. S2.
The prevalence of cercarial infection was assessed as described by Bush et al. [
26]. The Chi-square test and odds ratios were used to compare the prevalence between the dry and wet seasons using R base functions. In addition, the exact confidence intervals (CIs) of the prevalences in the 2 seasons were calculated as previously described [
27] using the epiR package [
28]. These statistical evaluations were executed in R for Windows v4.3.2 (R Foundation for Statistical Computing, Vienna, Austria) through RStudio v2023.12.1 Build 402 (Posit PBC, Boston, MA, USA).
Results
General status of cercarial infection in freshwater snails
In a survey of 35 sites across all administrative zones in the BMR canal network, 29,420 freshwater snails were collected from January 2018 to October 2019, spanning two cycles in the wet and dry seasons. The snail specimens belonged to 11 families, 21 genera, and 24 species/subspecies (
Supplementary Table S1;
Fig. 2). Among them, 1,275 snails belonging to 12 species/subspecies (
Supplementary Table S1;
Table 2) were infected with cercariae, revealing an overall prevalence of 4.3%.
Variabilities in the prevalence of cercarial infection were evident across the 35 surveyed sites (
Table 1). At the provincial level, the highest mean prevalence of cercarial infections in freshwater snails (5.7%; range, 0.0–4.6%) was observed in Pathum Thani, followed by Nakhon Pathom (5.1%; 1.6–8.4%), Nonthaburi (4.2%; 0.2–6.8%), Samut Sakhon (3.1%; 0.0–6.5%), Bangkok (2.5%; 0.0–4.6%), and Samut Prakan (2.2%; 0.0–5.5%). The highest prevalence (12.4%; 133/1,072) was observed at the Ra Haeng Canal in Lat Lum Kaew, Pathum Thani (PT6). In contrast, no infections were recorded at 4 surveyed sites: BK3, SP1, SP3, and SS3 (
Table 1).
Among the 12 field-infected freshwater snail species/subspecies identified in this study,
M. tuberculata exhibited the highest prevalence of cercarial infection at 9.9% (62/624), while the lowest prevalence was observed in
Tarebia granifera at 0.2% (7/4,591) (
Table 2). Despite its widespread occurrence across 27 sites,
M. tuberculata hosted a limited cercariae diversity with only 4 morphologically distinct types across 3 morphotypes. The snail species was moderately frequent within the BMR canal network (detected in 13 sites per sampling period), with a nearly equal ratio of sites with and without cercarial infections (13:14). On the other hand, the
B. siamensis siamensis detected in 31 sites, showed the second-highest prevalence rate of infection at 8.5% (743/8,741); additionally, it hosted the greatest diversity of cercariae with 15 morphologically distinct types identified across 11 morphotypes, accounting for up to 40.5% of the total number of distinct cercarial types (
Table 2;
Supplementary Table S1;
Figs. 3,
4).
B. siamensis siamensis also demonstrated a high occurrence frequency, averaging 23 sites per sampling period (range, 20–27 sites). Notably,
B. siamensis siamensis exhibited a significantly higher proportion of infected sites (23) than uninfected sites (8), accounting for 74.2% of the total number of sites where it was detected.
A total of 37 morphologically distinct types across 15 morphotypes of cercariae (
Table 3;
Figs. 3,
4) were detected from all the snail specimens. Fifteen morphotypes of cercariae were classified up to the family level, encompassing a range of 15–19 families, as listed in
Table 3. Among the 15 cercarial morphotypes, armatae xiphidiocercariae (
Figs. 3R–V, 4W–Y) was most prevalent (infection rate, 1.8%; 526/29,420), exhibited the greatest diversity (8 morphologically distinct types) within a morphotype of cercariae (
Table 3), and encompassed the broadest snail host spectrum (7 species/subspecies:
B. siamensis siamensis,
Wattebledia siamensis,
Radix rubiginosa,
M. tuberculata,
T. granifera,
Filopaludina martensi martensi, and
F. sumatrensis polygramma) (
Table 2;
Supplementary Table S1). The mutabile cercariae (
Fig. 3A, B) and virgulate xiphidiocercariae (
Fig. 3P) morphotypes were the second and third most prevalent at 0.9% (258/29,420) and 0.8% (228/29,420), respectively, (
Table 3). The combination of these 3 morphotypes represented 77.7% of the overall prevalence of cercarial infection in freshwater snails from the canal network in the BMR. In contrast, 5 cercarial morphotypes, i.e., clinostomatoid cercariae (
Fig. 3F, G), lophocercous-apharyngeate cercariae (
Fig. 3H, I), ubiquita xiphidiocercaria (
Fig. 3Q), megalurous cercaria (
Fig. 4f), and pleurolophocercous cercariae (
Fig. 4g, h), displayed minimal occurrences, each with a prevalence of less than 0.03% (
Table 3). Additionally, they displayed a low cercarial diversity (1–2 morphologically distinct types) within morphotypes (
Table 3) and a limited host snail spectrum (range, 1–2 species/subspecies;
Table 2).
Dichotoma cercaria (
Fig. 3E;
Supplementary Fig. S2), a cercarial morphotype of marine trematodes belonging to the Gymnophallidae family, was discovered from 4 freshwater snail taxa, i.e.,
W. siamensis,
B. siamensis siamensis,
F. martensi martensi, and
F. sumatrensis polygramma, with a prevalence of 0.1% (1/792), 0.1% (12/8,841), 0.4% (15/3,504), and 0.3% (12/4,792), respectively (
Supplementary Table S1). The prevalence of this cercarial morphotype across all snail samples was 0.1% (40/29,420), with a higher prevalence during the wet season (0.2%; 38/16,473) than in the dry season (0.02%; 2/12,947). Dichotoma cercaria was found to be distributed in nine sites, with the highest prevalence observed in NB4 (1.5%; 18/1,191) and the lowest in NP5 and NB6 (0.1%; 1/923) (
Supplementary Table S2;
Supplementary Fig. S1).
Examination of the general morphology of the dichotoma cercaria revealed an elongated oval body with a forked, longifurcate tail that was shorter than the body. The tail stem was shaped like a large, plump rod, with the tail furcae accounting for approximately 3-quarters of its length. Both the oral and ventral suckers were nearly equal in size, the former being pear-shaped and large. The ventral sucker, positioned in the third region of the body from the anterior end, appeared large, muscular, and highly noticeable, with a wide opening that accounted for about 3-quarters of its size. The prepharynx was present, and the pharynx was large and distinctly muscular, featuring a prominent medial slit bordered by distinct ridges on both sides. The esophagus and intestinal caeca were short and located in the middle of the body. The penetration glands were large and spherical, comprising one pair, each flanking the pharynx and the esophagus, with their large tubes entering on both sides of the base of the oral sucker. The excretory bladder was notably large, U-shaped, and connected to the proximal end of the tail. The excretory duct appeared in the middle region of the tail stem and bifurcated at the tail furcae. The excretory pores were prominent and situated at the tip of the tail furcae. The 23 metric morphological traits of the dichotoma cercaria are presented in
Supplementary Table S3 and
Supplementary Fig. S2.
The total prevalence of cercariae in freshwater snails from the BMR canal network was notably higher during the wet season (2.4%; 305/12,947; exact CIs=5.5–6.3%) compared to that in the dry season (5.9%; 970/16,473; exact CIs=2.3–3.0%). This difference was statistically significant, as confirmed by a Chi-square value (χ
2) of 218.21 (degrees of freedom [df], 1;
P-value<0.001). Moreover, the calculated odds ratio was 2.59 (95% CI, 2.28–2.96), indicating a 2.6 times higher risk of infection during the wet season compared to the dry season. Overall, higher prevalences of almost all morphotypes of cercariae were generally detected in the wet season (
Table 3), with a mean prevalence of 0.4% (range, 0.00–2.4%), while a mean prevalence of 0.2% (range, 0.0–1.0%) was detected in the dry season. Two exceptions were noted: pleurolophocercous cercariae and parapleurolophocercous cercariae displayed slightly higher prevalences in the dry season, and ubiquita cercaria and clinostomatoid cercariae were found exclusively in 2 individual snail samples during the dry season.
Discussion
In the present study, the overall prevalence of cercarial infections in freshwater snails from the BMR canal network was 4.3%, a finding slightly divergent from previous investigations in central Thailand; a lower overall prevalence of 3.3% for cercarial infection in freshwater snails from natural ponds and canals in Bangkok [
14]. This variation in infection rates could be partially attributed to the unceasing decrease in suitable habitats for potential intermediate and definitive hosts of certain digenean trematodes [
29–
31] in Bangkok compared to those in the broader BMR. In contrast, earlier studies [
9,
11] documented overall prevalences of 4.7% and 5.9% for cercarial infection in freshwater snails from Nakhon Nayok and the Chao Phraya Basin, respectively, regions characterized by lower urbanization levels compared to the BMR [
32].
Melanoides tuberculata showed the highest cercarial infection rate (9.9%) among the 24 snail species/subspecies examined within the BMR canal network. This observation is consistent with a previous report [
10], wherein a cercarial infection rate of 5.5% was observed in
M. tuberculata from 11 freshwater snail species in various aquatic environments in the Mae Lao Agricultural Basin at Chiang Rai, Thailand. A previous investigation [
12] also documented a higher infection rate of 18.8% in
M. tuberculata across Thailand, suggesting a pronounced vulnerability of this snail species to larval trematode infections.
Despite the highest cercarial infection rates in M. tuberculata in the current and prior studies, our results indicate that the B. siamensis siamensis snail may represent a greater potential risk to public health, livestock husbandries, and aquacultures. This assessment was based on 5 key factors exhibited by B. siamensis siamensis: (1) a marginally lower infection rate of 8.5% compared to 9.9% in M. tuberculata; (2) a more extensive geographic presence, detected in 31 sites as opposed to 27 for M. tuberculata; (3) a generally higher occurrence frequency, with B. siamensis siamensis detected in an average of 23 sites per sampling period (range, 20–27 sites) compared to an average of 13 sites for M. tuberculata (range, 7–19 sites); (4) a higher ratio of infected to uninfected sites (23:8) compared to M. tuberculata (13:14); and (5) a markedly greater diversity of hosted trematode cercariae, harboring 15 morphologically distinct types across 11 morphotypes, in contrast to M. tuberculata, which hosted 4 distinct types within 3 morphotypes.
Among 11 cercarial morphotypes from
B. siamensis siamensis snails, classified into 11–15 digenean families, members of 10 families have been associated with varying degrees of public health, veterinary, and socio-economic concerns. For instance, massive mortalities in farmed Nile tilapia fish were linked to Cyathocotylidae spp. and motile aeromonad co-infections [
5]. Aporocotylidae, affecting both wild and farmed marine fish, hold economic importance; however, the economic impact has not been reported in Thailand so far [
3]. Certain species of schistosomatidae are critical due to their roles in widespread schistosomiasis, affecting humans and livestock globally, especially in tropical regions [
1,
4]. Philopthalmidae occasionally infect humans; a rare case of conjunctival philophthalmiasis was reported in Thailand [
33]. Opisthorchiidae, especially
Opisthorchis viverrini sensu lato, with tobacco-pipe-shaped cercariae morphologically similar to pleurolophocercous cercaria I, are medically significant in Thailand [
8]. Heterophyids are capable of causing severe clinical manifestations when they accidentally travel to vital organs [
34]. These aspects underscore the significant ecological and epidemiological implications of
B. siamensis siamensis within the BMR.
In Thailand, earlier investigations on trematode cercariae in freshwater snails revealed a low to moderate diversity, with recorded instances of 5 to 9 morphotypes and 6 to 9 distinct types across the examined 6 to 20 snail species/subspecies [
6,
9–
11,
14,
35]. In stark contrast, the current study uncovered an obviously higher diversity of trematode cercariae, documenting 37 morphologically distinct types across 15 morphotypes from 24 freshwater snail species/subspecies. This marked increase plausibly aligns with the findings of Gordy et al. [
36], who demonstrated a positive correlation between the diversities of trematode communities and snail species.
Among 15 cercarial morphotypes classified across 15–19 digenean families, the armatae morphotype (Plagiorchiidae/Telorchiidae) recorded the highest infection rate at 1.8%, constituting 40.5% of the total infections and exhibiting the greatest diversity with 8 distinct types, likely due to the broadest host range (7 snail species/subspecies) [
36]. The small size of the armatae morphotype may enhance larval concentrations within intermediate hosts, boosting the transmission potential [
37]. Additionally, its stylet facilitates host penetration, increasing infection efficacy. Moreover, plagiorchiids, which have a broad definitive host range spanning all vertebrate classes and utilize freshwater snails, fishes, and diverse aquatic insects as second intermediate hosts [
1], along with telorchiids, which target amphibians and reptiles as definitive hosts and use snails, bivalves, and tadpoles as second intermediate hosts [
1], likely promote interactions among the life cycle stages and their compatible hosts within shared ecosystems, enhancing infection opportunities. Regarding the impact on human health, only 5
Plagiorchis species have been reported as human parasites, and their pathological effects are considered minimal [
34].
Interestingly, the current study documented the first instance of a gymnophallid digenean in freshwater, identifying dichotoma cercaria in freshwater
F. martensi martensi,
F. sumatrensis polygramma,
B. siamensis siamensis, and
Wattebledia siamensis snails in Thailand. Gymnophallidae, a small marine digenean family, primarily utilizes marine bivalves as first and second intermediate hosts, although some members in the metacercarial stage do not encyst and rarely parasitize marine gastropods, brachiopods, and polychaetes [
1,
38]. Notably,
Gymnophalloides seoi, commonly found in the Palearctic oystercatcher (
Haematopus ostralegus), also poses health risks to humans, particularly in endemic coastal areas of South Korea, where consumption of raw or undercooked oysters is linked to gastrointestinal issues ranging from mild to severe symptoms [
39].
In the present study, the cercarial infection rate in freshwater snails was significantly higher during the wet season (5.9%) than in the dry season (2.4%), exhibiting a 2.5-fold increase. This pattern was consistent across most cercarial morphotypes and aligns with previous findings by Brockelman et al. [
40], who noted that seasonal factors like the water temperature and duration and amount of rainfall significantly influence the interactions between hosts and parasites. A previous study has shown that the rainy and monsoon seasons enhance digenean transmission because increased precipitation can lead to higher fecal contamination with digenean eggs from definitive and reservoir hosts to snail habitats [
21].
In conclusion, 37 morphologically distinct types across 15 morphotypes of cercariae were detected in 12 of the 24 examined species/subspecies of freshwater snails from canal networks in the BMR, Thailand. Among all freshwater snails identified, the Bithynia siamensis siamensis subspecies exhibited the most significant epidemiological role in snail-transmitted parasitic diseases within the BMR flowing-water networks. The prevalence of cercariae in freshwater snails varied seasonally. The present study is the first to document the presence of gymnophallid larval fluke (dichotoma cercaria) in freshwater intermediate hosts. Additionally, this study provides qualitative and quantitative morphological descriptions and measurement guidelines for the newly discovered gymnophallid cercaria. Nonetheless, molecular approaches and phylogenetic analyses are essential for precisely identifying this cercaria species in the future.
Notes
-
Author contributions
Conceptualization: Rachprakhon P, Purivirojkul W
Data curation: Rachprakhon P
Formal analysis: Rachprakhon P
Funding acquisition: Purivirojkul W
Investigation: Rachprakhon P
Methodology: Rachprakhon P
Project administration: Purivirojkul W
Software: Rachprakhon P
Writing – original draft: Rachprakhon P
Writing – review & editing: Rachprakhon P, Purivirojkul W
-
The authors declare that there are no conflicts of interest associated with this article.
Supplementary Information
Acknowledgment
This research is funded by Kasetsart University through the Graduate School Fellowship Program, Kasetsart University Research and Development Institute (KURDI), International SciKU Branding (ISB), Faculty of Science, Kasetsart University, Department of Zoology, Faculty of Science and Kasetsart University through the Biodiversity Center, Kasetsart University.
Fig. 1A map depicting 35 surveyed sites within the canal network of the BMR, Thailand, with detailed information (code, river system and administrative area) on each location provided in
Table 1.
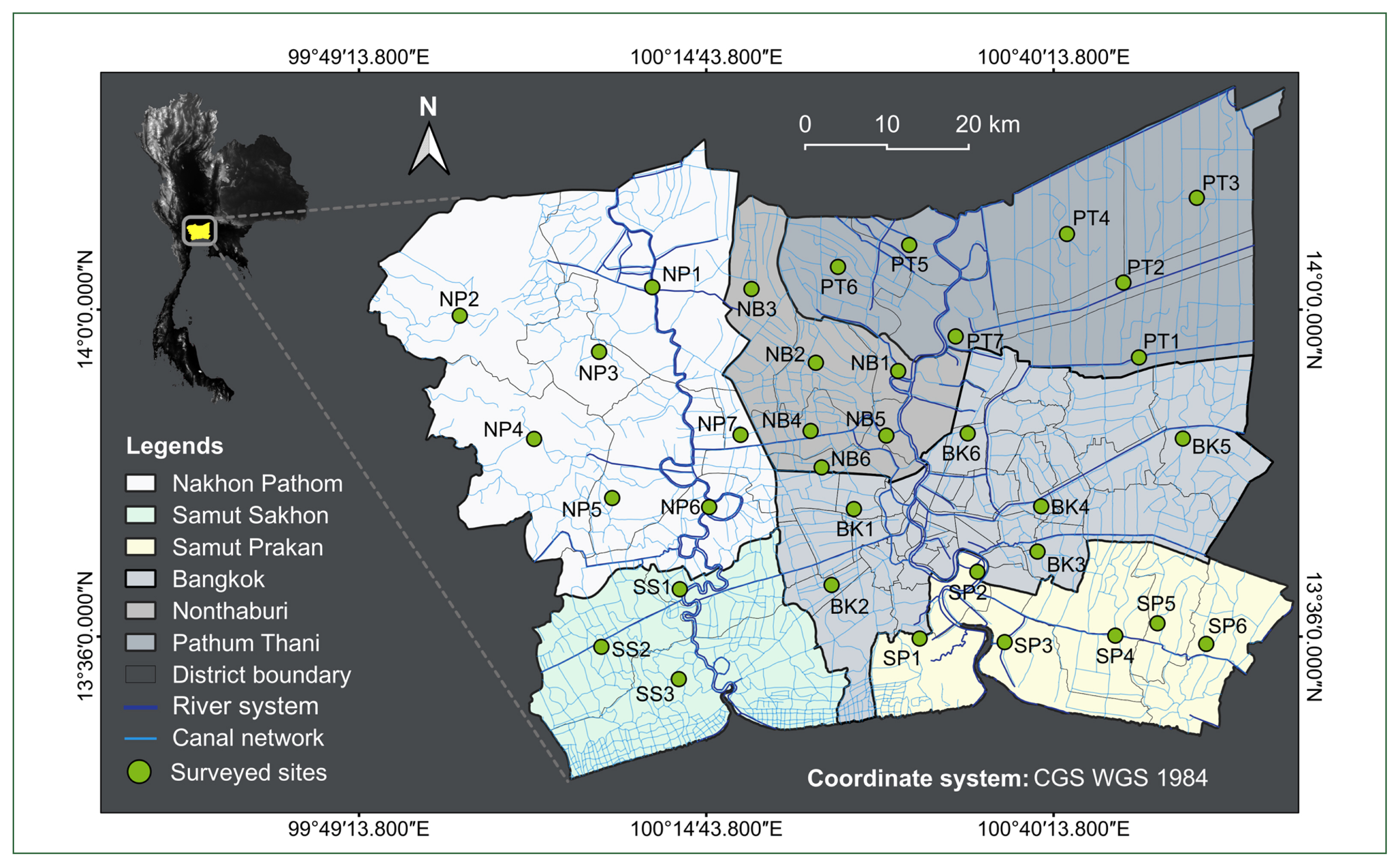
Fig. 2Freshwater snail specimens from the canal network system of the BMR, Thailand; (A) Pomacea canaliculata, (B) Pila gracilis, (C) Bithynia siamensis siamensis, (D) Wattebledia siamensis, (E) Filopaludina martensi martensi, (F) Filopaludina sumatrensis polygramma, (G) Filopaludina sumatrensis speciosa, (H) Idiopoma umbilicata, (I) Indoplanorbis exustus, (J) Gyrualus siamensis, (K) Polypylis hemisphaerula, (L) Amerianna carinata, (M) Radix rubiginosa, (N) Austropeplea viridis, (O) Physella acuta, (P–Q) Anentome helena, (R) Sulcospira housei, (S) Melanoides tuberculata, (T) Melanoides jugicostis, (U) Thiara scabra, (V) Tarebia granifera, (W) Sermyla riqueti, (X) Rehderiella parva, (Y) Cyclotropis carinata. Scale bar for A–B, E–H, and P–W=10 mm. Scale bar for C–D, I–O, and X–Y=5 mm.

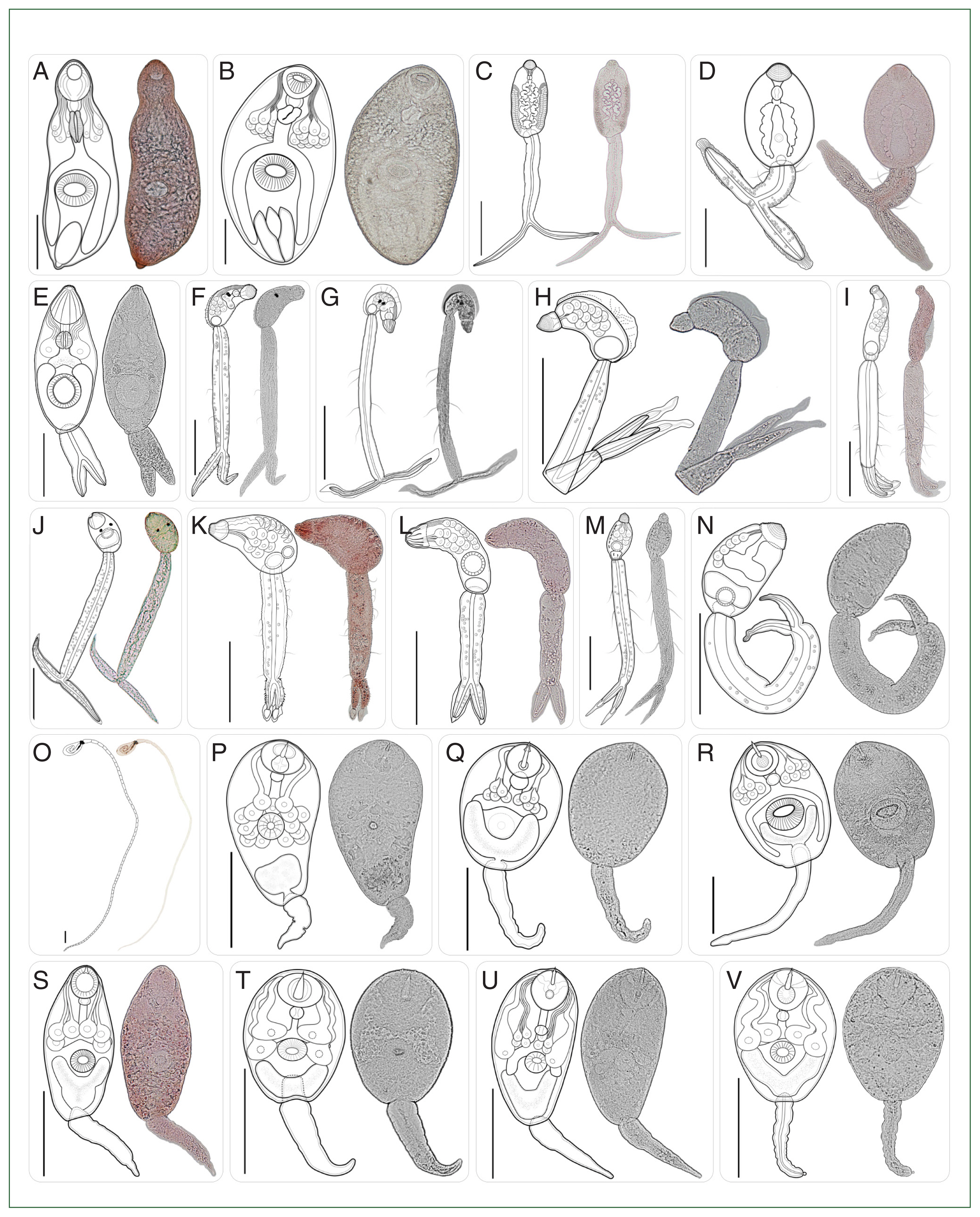
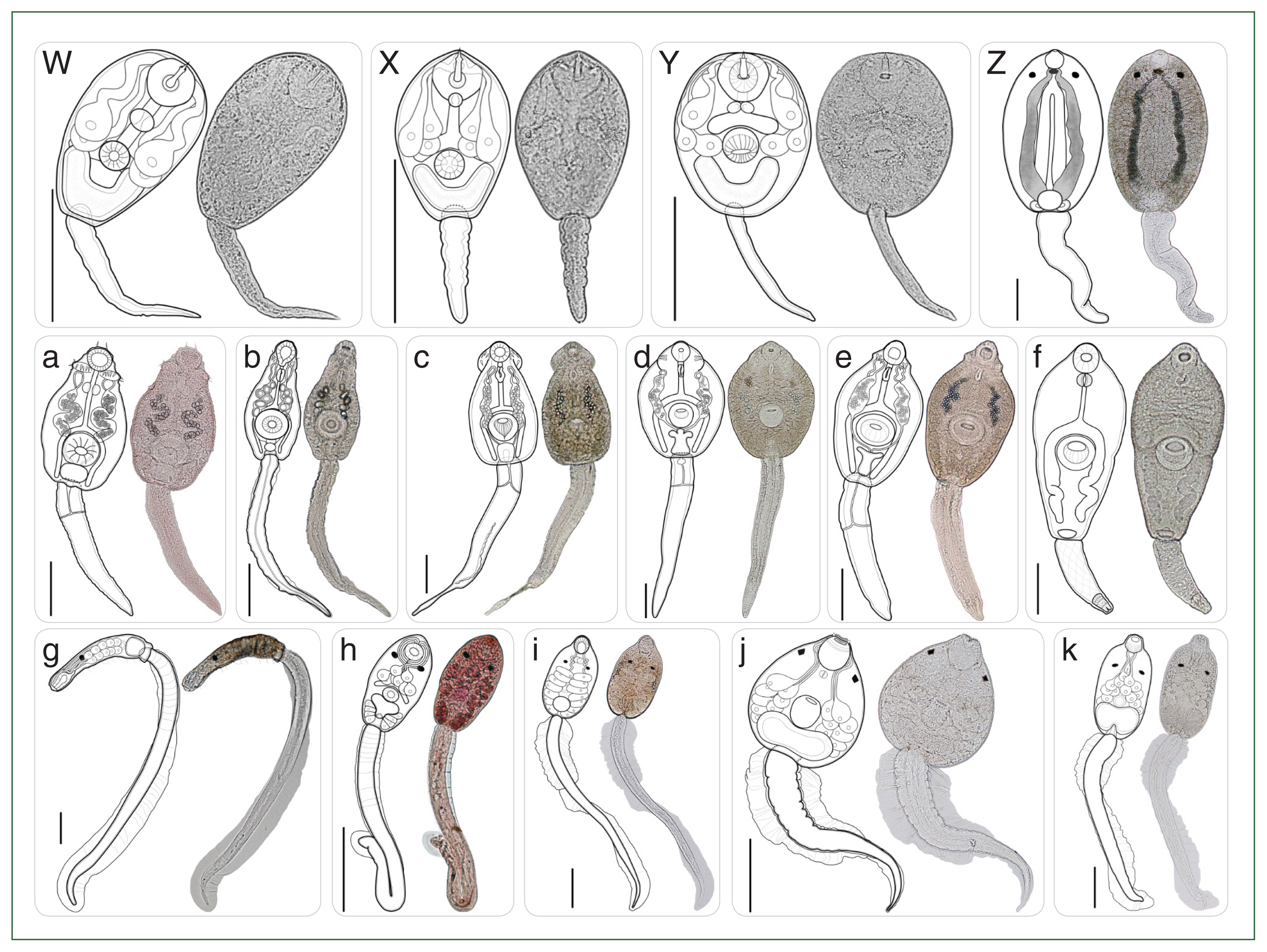
Fig. 3Type of cercariae infecting freshwater snails obtained from the canal network of the BMR, Thailand: Part 1 (Morphologically distinct 22 types). (A–B) Mutabile cercariae I–II; (C–D) vivax cercariae I–II; (E) dichotoma cercaria; (F–G) brevifurcate-pharyngeate clinostomatoid cercariae I–II; (H–I) lophocercous-apharyngeate cercariae I–II; (J–N) brevifurcate-apharyngeate cercariae I–V; (O) cystophorous cercaria; (P) virgulate xiphidiocercaria; (Q) ubiquita cercaria; (R–Y) armatae xiphidiocercariae I–V. Scale bar=100 μm.
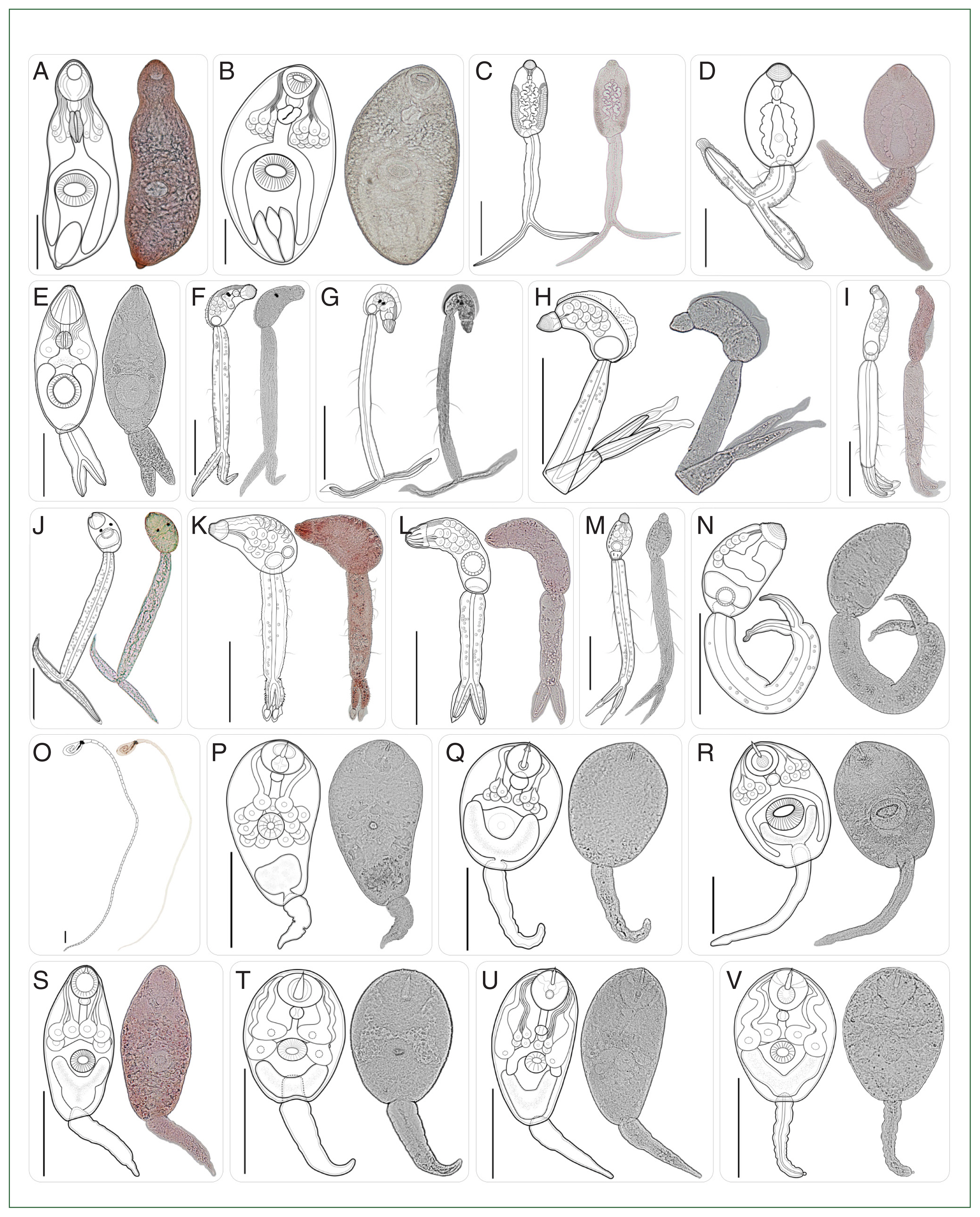
Fig. 4Type of cercariae infecting freshwater snails obtained from the canal network of the BMR, Thailand: Part 2 (Morphologically distinct 15 types). (W–Y) Armatae xiphidiocercariae VI–VIII; (Z) monostome cercaria; (a–e) echinostome cercariae I–V; (f) megalurous cercaria; (g–h) pleurolophocercous cercariae I–II; (i–k) parapleurolophocercous cercariae I–III. Scale bar=100 μm.
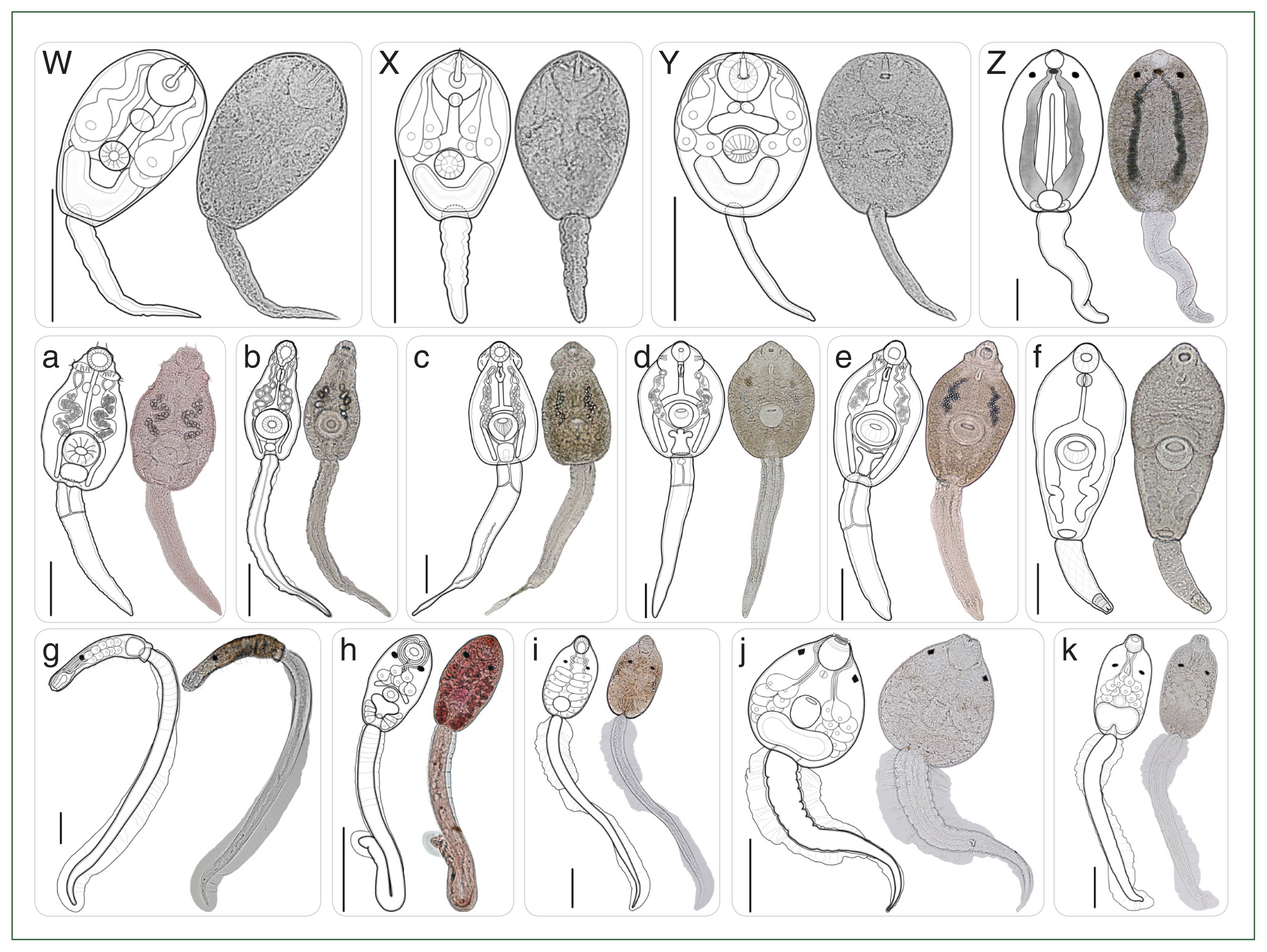
Table 1Cercarial infection status in freshwater snails by the 35 surveyed sites
Table 1
|
Surveyed sites |
NSEa
|
NI/NE (%)b
|
|
No. |
Code |
Province |
Zone (district) |
River system |
Khlong (canal) |
|
1 |
BK1 |
Bangkok |
North Thonburi |
Chao Phraya |
Bang Phrom’s branch |
12 |
48/1,045 (4.6) |
|
2 |
BK2 |
Bangkok |
South Thonburi |
Chao Phraya |
Bang Phran |
10 |
9/386 (2.3) |
|
3 |
BK3 |
Bangkok |
South Bangkok |
Chao Phraya |
Phra Khanong |
6 |
0/331 (0.0) |
|
4 |
BK4 |
Bangkok |
Central Bangkok |
Chao Phraya |
Hua Mak |
7 |
4/612 (3.0) |
|
5 |
BK5 |
Bangkok |
East Bangkok |
Chao Phraya |
Lam Jian Dub |
13 |
31/1,028 (3.0) |
|
6 |
BK6 |
Bangkok |
North Bangkok |
Chao Phraya |
KU canalc
|
10 |
24/1,060 (2.3) |
|
7 |
NB1 |
Nonthaburi |
Pak Kret |
Chao Phraya |
Phra Udom |
14 |
9/319 (2.8) |
|
8 |
NB2 |
Nonthaburi |
Bang Bua Thong |
Chao Phraya |
Phra Phimon |
13 |
26/512 (5.1) |
|
9 |
NB3 |
Nonthaburi |
Sai Noi |
Chao Phraya |
Khun Si |
10 |
42/1,177 (3.6) |
|
10 |
NB4 |
Nonthaburi |
Bang Yai |
Chao Phraya |
Charoen Suk |
9 |
76/1,191 (6.4) |
|
11 |
NB5 |
Nonthaburi |
Mueang |
Chao Phraya |
Bang Krang |
16 |
2/1,026 (0.2) |
|
12 |
NB6 |
Nonthaburi |
Bang Kruai |
Chao Phraya |
Plai Bang |
13 |
63/923 (6.8) |
|
13 |
NP1 |
Nakhon Pathom |
Bang Len |
Tha Chin |
Phra Phimon |
8 |
21/423 (5.0) |
|
14 |
NP2 |
Nakhon Pathom |
Kamphaeng Saen |
Tha Chin |
Mon Thong |
7 |
23/283 (8.1) |
|
15 |
NP3 |
Nakhon Pathom |
Don Tum |
Tha Chin |
Ban Yen |
12 |
17/641 (2.7) |
|
16 |
NP4 |
Nakhon Pathom |
Mueang |
Tha Chin |
Chedi Bucha |
10 |
76/901 (8.4) |
|
17 |
NP5 |
Nakhon Pathom |
Nakhon Chai Sri |
Tha Chin |
Bang Rakam |
11 |
50/1,799 (2.8) |
|
18 |
NP6 |
Nakhon Pathom |
Sam Phran |
Tha Chin |
Lat Nang Than |
10 |
32/435 (7.4) |
|
19 |
NP7 |
Nakhon Pathom |
Phutthamonthon |
Tha Chin |
Ta Khui |
13 |
9/582 (1.6) |
|
20 |
PT1 |
Pathum Thani |
Lam Luk Ka |
Chao Phraya |
Hok Wa Sai Lang |
10 |
79/1,513 (5.2) |
|
21 |
PT2 |
Pathum Thani |
Thanyaburi |
Chao Phraya |
Chet |
9 |
66/830 (8.0) |
|
22 |
PT3 |
Pathum Thani |
Nong Sua |
Chao Phraya |
Sip Et Tributary |
8 |
12/429 (2.9) |
|
23 |
PT4 |
Pathum Thani |
Khlong Luang |
Chao Phraya |
Si |
8 |
150/1,502 (10.0) |
|
24 |
PT5 |
Pathum Thani |
Sam Khok |
Chao Phraya |
Khut |
10 |
5/443 (1.1) |
|
25 |
PT6 |
Pathum Thani |
Lat Lum Kaew |
Chao Phraya |
Ra Haeng |
10 |
133/1,072 (12.4) |
|
26 |
PT7 |
Pathum Thani |
Mueang |
Chao Phraya |
Rangsit Prayurasakdi |
11 |
1/459 (0.2) |
|
27 |
SP1 |
Samut Prakan |
Phra Samut Chedi |
Chao Phraya |
Ka Om Nai |
7 |
0/1,153 (0.0) |
|
28 |
SP2 |
Samut Prakan |
Phra Pradaeng |
Chao Phraya |
Bang Namphueng |
12 |
3/1,375 (0.2) |
|
29 |
SP3 |
Samut Prakan |
Mueang |
Chao Phraya |
Bang Ping |
7 |
0/186 (0.0) |
|
30 |
SP4 |
Samut Prakan |
Bang Phli |
Chao Phraya |
Samrong |
10 |
21/590 (3.6) |
|
31 |
SP5 |
Samut Prakan |
Bang Saothong |
Chao Phraya |
Chorakhe Yai |
9 |
72/1,318 (5.5) |
|
32 |
SP6 |
Samut Prakan |
Bang Bo |
Chao Phraya |
Chuat Phrao |
15 |
35/871 (4.0) |
|
33 |
SS1 |
Samut Sakhon |
Krathum Baen |
Tha Chin |
Naew Niyom’s branch |
12 |
25/903 (2.8) |
|
34 |
SS2 |
Samut Sakhon |
Ban Phaew |
Tha Chin |
Rang Mor Kaeng |
11 |
111/1,699 (6.5) |
|
35 |
SS3 |
Samut Sakhon |
Mueang |
Tha Chin |
Khan Phanang |
11 |
0/413 (0.0) |
|
Overall total |
24 |
1,275/29,420 (4.3) |
Table 2Infection status and diversity of cercariae in freshwater snails
Table 2
|
Snail species/subspecies |
NI/NE (%)a
|
No. site detected (average OFSb: range OFS) |
IS: UIS (%)c
|
NMd (NMDe) |
Cercariae harbored by snail (see codes in Table 3 and Figs. 3, 4) |
|
Bithyniidae |
|
Bithynia siamensis siamensis
|
743/8,741 (8.5) |
31 (23: 20–27) |
23:8 (74) |
11 (15) |
B, D, E, I, K, L, M, O, P, S, X, Y, Z, g, j |
|
Wattebledia siamensis
|
9/792 (1.1) |
14 (6: 1–14) |
5:9 (36) |
3 (5) |
E, S, U, X, j |
|
|
Viviparidae |
|
Filopaludina martensi martensi
|
235/3,504 (6.7) |
34 (23: 19–27) |
26:8 (76) |
4 (5) |
C, E, S, U, d |
|
Filopaludina sumatrensis polygramma
|
175/4,792 (3.7) |
32 (22: 18–27) |
25:7 (78) |
5 (7) |
C, D, E, H, T, U, c |
|
Filopaludina sumatrensis speciosa
|
1/15 (6.7) |
7 (1: 0–3) |
1:6 (14) |
1 (1) |
C |
|
|
Planorbidae |
|
Indoplanorbis exustus
|
1/124 (0.8) |
18 (5: 2–9) |
1:17 (6) |
1 (1) |
b |
|
|
Lymnaeidae |
|
Radix rubiginosa
|
12/421 (2.9) |
29 (11: 6–19) |
8:21 (28) |
3 (5) |
F, G, R, a, e |
|
|
Nassariidae |
|
Anentome helena
|
9/890 (1.0) |
21 (13: 11–15) |
4:17 (19) |
2 (4) |
A, J, L, N |
|
|
Pachychilidae |
|
Sulcospira housei
|
7/156 (4.5) |
5 (2: 0–5) |
1:4 (20) |
1 (1) |
f |
|
|
Thiaridae |
|
Melanoides tuberculata
|
62/624 (9.9) |
27 (13: 7–19) |
13:14 (48) |
3 (4) |
V, Q, i, k |
|
Melanoides jugicostis
|
6/82 (7.3) |
4 (1: 0–3) |
1:3 (25) |
2 (2) |
K, h |
|
Tarebia granifera
|
7/4,591 (0.2) |
24 (12: 9–16) |
2:22 (8) |
2 (3) |
V, W, i |
Table 3Seasonal infection status of 37 morphologically distinct types across 15 morphotypes of trematode cercariae
Table 3
|
Type of cercariae |
Code |
Trematode taxon |
SHRa
|
No. snail infected (%) |
|
|
Dry seasonb
|
Wet seasonc
|
Totald
|
|
Mutabile cercariae |
A–B |
Lissorchiidae |
2 |
56 (0.4) |
202 (1.2) |
258 (0.9) |
|
Mutabile cercaria I |
A |
1 |
1 (0.01) |
3 (0.02) |
4 (0.01) |
|
Mutabile cercaria II |
B |
1 |
55 (0.4) |
199 (1.2) |
254 (0.9) |
|
|
Vivax cercariae |
C–D |
Cyathocotylidae |
4 |
5 (0.04) |
18 (0.11) |
23 (0.1) |
|
Vivax cercaria I |
C |
2 |
4 (0.03) |
3 (0.02) |
7 (0.02) |
|
Vivax cercaria II |
D |
3 |
1 (0.01) |
15 (0.1) |
16 (0.1) |
|
|
Dichotoma cercaria |
E |
Gymnophallidae |
4 |
2 (0.02) |
38 (0.2) |
40 (0.1) |
|
|
Clinostomatoid cercariae |
F–G |
Clinostomidae |
1 |
2 (0.02) |
0 (0.00) |
2 (0.01) |
|
Clinostomatoid cercariae I |
F |
1 |
1 (0.01) |
0 (0.00) |
1 (0.003) |
|
Clinostomatoid cercariae II |
G |
1 |
1 (0.01) |
0 (0.00) |
1 (0.003) |
|
|
Lophocercous-apharyngeate cercariae |
H–I |
Aporocotylidae |
2 |
0 (0.00) |
4 (0.02) |
4 (0.01) |
|
Lophocercous-apharyngeate cercariae I |
H |
1 |
0 (0.00) |
2 (0.01) |
2 (0.01) |
|
Lophocercous-apharyngeate cercariae II |
I |
1 |
0 (0.00) |
2 (0.01) |
2 (0.01) |
|
|
Brevifurcate-apharyngeate cercariae |
J–N |
Schistosomatidae/Spirochiidae |
3 |
13 (0.1) |
30 (0.2) |
43 (0.2) |
|
Brevifurcate-apharyngeate cercariae I |
J |
1 |
0 (0.00) |
1 (0.01) |
1 (0.003) |
|
Brevifurcate-apharyngeate cercariae II |
K |
2 |
5 (0.04) |
1 (0.01) |
6 (0.02) |
|
Brevifurcate-apharyngeate cercariae III |
L |
2 |
0 (0.00) |
10 (0.1) |
10 (0.03) |
|
Brevifurcate-apharyngeate cercariae IV |
M |
1 |
8 (0.06) |
15 (0.1) |
23 (0.1) |
|
Brevifurcate-apharyngeate cercariae V |
N |
1 |
0 (0.00) |
3 (0.02) |
3 (0.01) |
|
|
Cystophorous cercaria |
O |
Hemiuridae |
1 |
7 (0.1) |
38 (0.23) |
45 (0.2) |
|
|
Virgulate xiphidiocercaria |
P |
Lecithodendriidae |
1 |
52 (0.4) |
176 (1.1) |
228 (0.8) |
|
|
Ubiquita xiphidiocercaria |
Q |
Microphallidae |
1 |
0 (0.0) |
1 (0.01) |
1 (0.003) |
|
|
Armatae xiphidiocercariae |
R–Y |
Plagiorchiidae/Telorchiidae |
7 |
129 (1.0) |
397 (2.4) |
526 (1.8) |
|
Armatae xiphidiocercaria I |
R |
1 |
0 (0.0) |
1 (0.01) |
1 (0.003) |
|
Armatae xiphidiocercaria II |
S |
3 |
22 (0.2) |
84 (0.5) |
106 (0.4) |
|
Armatae xiphidiocercaria III |
T |
1 |
8 (0.1) |
2 (0.01) |
10 (0.03) |
|
Armatae xiphidiocercaria IV |
U |
3 |
83 (0.7) |
283 (1.7) |
366 (1.2) |
|
Armatae xiphidiocercaria V |
V |
3 |
8 (0.1) |
19 (0.1) |
27 (0.1) |
|
Armatae xiphidiocercaria VI |
W |
1 |
1 (0.01) |
0 (0.00) |
1 (0.003) |
|
Armatae xiphidiocercaria VII |
X |
2 |
5 (0.04) |
3 (0.02) |
8 (0.03) |
|
Armatae xiphidiocercaria VIII |
Y |
1 |
2 (0.02) |
5 (0.03) |
7 (0.02) |
|
|
Monostome cercaria |
Z |
Notocotylidae |
1 |
1 (0.01) |
14 (0.1) |
15 (0.1) |
|
|
Echinostome cercariae |
a–e |
Echinostomatidae |
4 |
2 (0.02) |
11 (0.1) |
13 (0.04) |
|
Echinostome cercaria I |
a |
1 |
0 (0.00) |
1 (0.01) |
1 (0.003) |
|
Echinostome cercaria II |
b |
1 |
0 (0.00) |
1 (0.01) |
1 (0.003) |
|
Echinostome cercaria III |
c |
1 |
0 (0.00) |
2 (0.01) |
2 (0.01) |
|
Echinostome cercaria IV |
d |
1 |
0 (0.00) |
1 (0.01) |
1 (0.00) |
|
Echinostome cercaria V |
e |
1 |
2 (0.02) |
6 (0.04) |
8 (0.03) |
|
|
Megalurous cercaria |
f |
Philophthalmidae |
1 |
0 (0.00) |
7 (0.04) |
7 (0.02) |
|
|
Pleurolophocercous cercariae |
g–h |
Opisthorchiidae/Heterophyidae/Cryptogonimidae |
2 |
3 (0.02) |
2 (0.01) |
5 (0.02) |
|
Pleurolophocercous cercaria I |
g |
1 |
2 (0.02) |
2 (0.01) |
4 (0.01) |
|
Pleurolophocercous cercaria II |
h |
1 |
1 (0.01) |
0 (0.00) |
1 (0.003) |
|
|
Parapleurolophocercous cercariae |
i–k |
Heterophyidae |
4 |
33 (0.3) |
32 (0.2) |
65 (0.2) |
|
Parapleurolophocercous cercaria I |
i |
2 |
22 (0.2) |
6 (0.04) |
28 (0.1) |
|
Parapleurolophocercous cercaria II |
j |
2 |
9 (0.1) |
16 (0.1) |
25 (0.1) |
|
Parapleurolophocercous cercaria III |
k |
1 |
2 (0.02) |
10 (0.1) |
12 (0.04) |
|
|
Total |
|
|
12 |
305 (2.4) |
970 (5.9*) |
1,275 (4.3) |
References
- 1. Schell SC. How to know the trematodes. Brown Company Publisher; Dubuque USA. 1970, p 354.
- 2. Chai JY, Jung BK. General overview of the current status of human foodborne trematodiasis. Parasitology 2022;149(10):1262-1285.
https://doi.org/10.1017/S0031182022000725
- 3. Paperna I, Dzikowski R. Digenea (Phylum Platyhelminthes). In Woo PTK ed, Fish Diseases and Disorders. Vol. 1: Protozoan and Metazoan Infections. CABI Publishing and the Natural History Museum; Wallingford, United Kingdom. 2006, pp 345-390.
- 4. World Health Organization. Schistosomiasis [Internet]; World Health Organization; Geneva, Switzerland: 2023. [updated 2023 Feb 1; cited 2024 Mar 12]. Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- 5. Younis NA, Elgendy MY, El-Samannoudy SI, Abdelsalam M, Attia MM. Cyathocotylidae spp. and motile aeromonads co-infections in farmed Nile tilapia (Oreochromis niloticus) causing mass mortality. Microb Pathog 2023;174:105897.
https://doi.org/10.1016/j.micpath.2022.105897
- 6. Krailas D, Namchote S, Komsuwan J, Wongpim T, Apiraksena K, et al. Cercarial dermatitis outbreak caused by ruminant parasite with intermediate snail host: schistosome in Chana, South Thailand. Evol Syst 2022;6:151-173.
https://doi.org/10.3897/evolsyst.6.87670
- 7. Agrawal MC. Prevalence in final host. In Agrawal MC ed, Schistosomes and Schistosomiasis in South Asia. Springer; New Delhi, India. 2012, pp 85-121.
- 8. Suwannatrai A, Saichua P, Haswell M. Epidemiology of Opisthorchis viverrini infection. In Sripa B, Brindley PJ eds, Advances in Parasitology. Academic Press; USA. 2018, pp 41-67.
- 9. Anucherngchai S, Tejangkura T, Chontananarth T. Epidemiological situation and molecular identification of cercarial stage in freshwater snails in Chao-Phraya Basin, Central Thailand. Asian Pac J Trop Biomed 2016;6(6):539-545.
https://doi.org/10.1016/j.apjtb.2016.01.015
- 10. Chantima K, Suk-ueng K, Kampan M. Freshwater snail diversity in Mae Lao Agricultural Basin (Chiang Rai, Thailand) with a focus on larval trematode infections. Korean J Parasitol 2018;56:247-257.
https://doi.org/10.3347/kjp.2018.56.3.247
- 11. Chontananarth T, Tejangkura T, Wetchasart N, Chimburut C. Morphological characteristics and phylogenetic trends of trematode cercariae in freshwater snails from Nakhon Nayok Province, Thailand. Korean J Parasitol 2017;55(1):47-54.
https://doi.org/10.3347/kjp.2017.55.1.47
- 12. Krailas D, Namchote S, Koonchornboon T, Dechruksa W, Boonmekam D. Trematodes obtained from the thiarid freshwater snail Melanoides tuberculata (Müller, 1774) as vector of human infections in Thailand. Zoosyst Evol 2014;90(1):57-86.
https://doi.org/10.3897/zse.90.7306
- 13. Veeravechsukij N, Namchote S, Neiber MT, Glaubrecht M, Krailas D. Exploring the evolutionary potential of parasites: larval stages of pathogen digenic trematodes in their thiarid snail host Tarebia granifera in Thailand. Zoosyst Evol 2018;94(2):425-460.
https://doi.org/10.3897/zse.94.28793
- 14. Wiroonpan P, Chontananarth T, Purivirojkul W. Cercarial trematodes in freshwater snails from Bangkok, Thailand: prevalence, morphological and molecular studies and human parasite perspective. Parasitology 2021;148(3):366-383.
https://doi.org/10.1017/S0031182020002073
- 15. Kiatsopit N, Sithithaworn P, Kopolrat K, Namsanor J, Andrews RH, et al. Trematode diversity in the freshwater snail Bithynia siamensis goniomphalos sensu lato from Thailand and Lao PDR. J Helminthol 2016;90(3):312-320.
https://doi.org/10.1017/S0022149X15000292
- 16. Kulsantiwong J, Prasopdee S, Piratae S, Khampoosa P, Thammasiri C, et al. Trematode infection of freshwater snails, family Bithyniidae in Thailand. Southeast Asian J Trop Med Public Health 2015;46(3):396-405.
- 17. Harkins B. Thailand Migration Report 2019. United Nations Thematic Working Group on Migration in Thailand; Bangkok, Thailand. 2019, p 194.
- 18. Molle F. The closure of the Chao Phraya River Basin in Thailand: its causes, consequences and policy implications. In Shivakoti GP, Vermillion DL, Lam WF, Ostrom E, Pradhan U, et al eds, Asian Irrigation in Transition: Responding to the Challenges Ahead. 2002, April. 22–23. Asian Institute of Technology; Bangkok, Thailand. Sage Publications; New Delhi, India. 2002, pp 1-15.
- 19. Thailand Rivers and Streams [Internet]. SavGIS; 2018. [cited 2019 Jan 20]. Available from: http://www.savgis.org/thailand.htm
- 20. Daniels RA. Untested assumptions: the role of canals in the dispersal of sea lamprey, alewife, and other fishes in the eastern United States. Environ Biol Fishes 2001;60(4):309-329.
https://doi.org/10.1023/A:1011032907484
- 21. Kaewkes W, Kaewkes S, Tesana S, Laha T, Sripa B. Fecal bacterial contamination in natural water reservoirs as an indicator of seasonal infection by Opisthorchis viverrini in snail intermediate hosts. Parasitol Int 2012;61(1):49-51.
https://doi.org/10.1016/j.parint.2011.08.013
- 22. Olivier L, Schneiderman M. A method for estimating the density of aquatic snail populations. Exp Parasitol 1956;5(2):109-117.
https://doi.org/10.1016/0014-4894(56)90008-X
- 23. Brandt RAM. The non-marine aquatic Mollusca of Thailand. Archiv für Molluskenkunde 1974;105(1–5):1-423.
- 24. Upatham ES, Sormani S, Kitikoon V, Lohachit C, Burch JB. Identification key for the fresh and brackish-water snails of Thailand. Malacol Rev 1983;16:107-132.
- 25. Wykoff DE, Harinasuta C, Juttijudata P, Winn MM.
Opisthorchis viverrini in Thailand: the life cycle and comparison with O. felineus. J Parasitol 1965;51(2):207-214.
https://doi.org/10.2307/3276083
- 26. Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997;83(4):575-583.
https://doi.org/10.2307/3284227
- 27. Reiczigel J, Marozzi M, Fábián I, Rózsa L. Biostatistics for parasitologists – a primer to quantitative parasitology. Trends Parasitol 2019;35(4):277-281.
https://doi.org/10.1016/j.pt.2019.01.003
- 28. Carstensen B, Plummer M, Laara E, Hills M. Epi: A Package for Statistical Analysis in Epidemiology [Software]. 2.48. The Comprehensive R Archive Network (CRAN): Bendix Carstensen; 20241 package: Extensions to the R statistical programming language.
- 29. McKinney ML. Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 2008;11(2):161-176.
https://doi.org/10.1007/s11252-007-0045-4
- 30. Gál B, Szivák I, Heino J, Schmera D. The effect of urbanization on freshwater macroinvertebrates – knowledge gaps and future research directions. Ecol Indic 2019;104:357-364.
https://doi.org/10.1016/j.ecolind.2019.05.012
- 31. Marcogliese DJ. Parasites of the superorganism: are they indicators of ecosystem health? Int J Parasitol 2005;35(7):705-716.
https://doi.org/10.1016/j.ijpara.2005.01.015
- 32. Karra K, Kontgis C, Statman-Weil Z, Mazzariello JC, Mathis M, et al. Global Land Use/Land cover with Sentinel 2 and Deep Learning. 2021 IEEE International Geoscience and Remote Sensing Symposium IGARSS. 2021 Jul 11–16; Brussels, Belgium: IEEE; 2021. 8707
https://doi.org/10.1109/IGARSS47720.2021.9553499
- 33. Waikagul J, Dekumyoy P, Yoonuan T, Praevanit R. Conjunctiva philophthalmosis: a case report in Thailand. Am J Trop Med Hyg 2006;74(5):848-849.
https://doi.org/10.4269/ajtmh.2006.74.848
- 34. Chai JY. Human Intestinal Flukes: from Discovery to Treatment and Control. Springer; Dordrecht, Netherlands. 2019, p 549.
- 35. Chontananarth T, Wongsawad C. Epidemiology of cercarial stage of trematodes in freshwater snails from Chiang Mai province, Thailand. Asian Pac J Trop Biomed 2013;3(3):237-243.
https://doi.org/10.1016/S2221-1691(13)60058-1
- 36. Gordy MA, Koprivnikar J, McPhail B, Hanington PC. Environmental and ecological factors driving trematode parasite community assembly in central Alberta lakes. Int J Parasitol Parasites Wildl 2020;13:283-291.
https://doi.org/10.1016/j.ijppaw.2020.11.008
- 37. Poulin R. Body size vs abundance among parasite species: positive relationships? Ecography 1999;22(3):246-250.
https://doi.org/10.1111/j.1600-0587.1999.tb00499.x
- 38. Cremonte F, Gilardoni C, Pina S, Rodrigues P, Ituarte C. Revision of the family Gymnophallidae Odhner, 1905 (Digenea) based on morphological and molecular data. Parasitol Int 2015;64(2):202-210.
https://doi.org/10.1016/j.parint.2014.12.003
- 39. Chai JY, Choi MH, Yu JR, Lee SH.
Gymnophalloides seoi: a new human intestinal trematode. Trends Parasitol 2003;19(3):109-112.
https://doi.org/10.1016/S1471-4922(02)00068-5
- 40. Brockelman WY, Upatham ES, Viyanant V, Ardsungnoen S, Chantanawat R. Field studies on the transmission of the human liver fluke, Opisthorchis viverrini, in northeast Thailand: population changes of the snail intermediate host. Int J Parasitol 1986;16(5):545-552.
https://doi.org/10.1016/0020-7519(86)90091-3